Time from last chemotherapy to death and its correlation with the end of life care in a referral hospital
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(01): 55-59
DOI: DOI: 10.4103/0971-5851.151792
Abstract
Background: A substantial number of cancer patients receive chemotherapy until the end of life (EoL). Various factors have been shown to be associated with receipt of chemotherapy until near death. In this study, we determine our average time from last chemotherapy to death (TLCD) and explore different factors that may be associated with decreased TLCD. Materials and Methods: A retrospective review of medical records of adult cancer patients who received chemotherapy during their illness and died in our hospital between January 2010 and January 2012 was conducted. Chi-square test and t-test were used to examine the correlation between selected factors and use of chemotherapy within 60 days of death. Multivariate analysis was used to test independent significance of factors testing positive in univariate analysis. Kaplan-Meier method was used to perform survival analysis. Results: Of the 115 cancer patients who died in the hospital, 41 (35.6%) had TLCD of 60 days or less. Patients with better performance status and those dying under medical oncology service were more likely to be in this group of patients. Univariate analysis showed that these patients were less likely to have palliative care involvement, were more likely to die of treatment related causes, and more likely to have died in the Intensive Care Unit. Multivariate analysis confirmed lack of palliative care involvement and better performance status as independent factors for TLCD less than 60 days. Survival analyses showed that patients with palliative care involvement and those dying under palliative care service were likely to have significantly longer TLCD. Conclusions: Cancer patients who have no involvement of palliative care team in their management tend to receive chemotherapy near the EoL, have more aggressive EoL care, and have higher risk of dying die from treatment related complications. Palliative care should be involved early in the care of cancer patients.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
A substantial number of cancer patients receive chemotherapy until the end of life (EoL). Various factors have been shown to be associated with receipt of chemotherapy until near death. In this study, we determine our average time from last chemotherapy to death (TLCD) and explore different factors that may be associated with decreased TLCD.
Materials and Methods:
A retrospective review of medical records of adult cancer patients who received chemotherapy during their illness and died in our hospital between January 2010 and January 2012 was conducted. Chi-square test and t-test were used to examine the correlation between selected factors and use of chemotherapy within 60 days of death. Multivariate analysis was used to test independent significance of factors testing positive in univariate analysis. Kaplan-Meier method was used to perform survival analysis.
Results:
Of the 115 cancer patients who died in the hospital, 41 (35.6%) had TLCD of 60 days or less. Patients with better performance status and those dying under medical oncology service were more likely to be in this group of patients. Univariate analysis showed that these patients were less likely to have palliative care involvement, were more likely to die of treatment related causes, and more likely to have died in the Intensive Care Unit. Multivariate analysis confirmed lack of palliative care involvement and better performance status as independent factors for TLCD less than 60 days. Survival analyses showed that patients with palliative care involvement and those dying under palliative care service were likely to have significantly longer TLCD.
Conclusions:
Cancer patients who have no involvement of palliative care team in their management tend to receive chemotherapy near the EoL, have more aggressive EoL care, and have higher risk of dying die from treatment related complications. Palliative care should be involved early in the care of cancer patients.
INTRODUCTION
Cancer afflicts 12,309 patients in Saudi Arabia every year. [1] As in the rest of the world, a substantial number of cancer patients in Saudi Arabia die in the hospital, a problem compounded by the lack of a structured community hospice system.
In-patient care near death in cancer patients not only accounts for majority of their cancer related costs, [2] but has also been associated with increased aggressiveness of care in these patients. [3] Available literature has identified many factors that are considered to be poor performance indicators in end of life (EoL) care. Chemotherapy near the EoL is one of these indicators and has been studied in various institutions internationally as a quality measure. [4,5,6,7,8]
In this study, we aim to explore factors associated with decreased time from last chemotherapy to death (TLCD) in cancer patients who died in our tertiary care referral hospital.
MATERIALS AND METHODS
After approval from our institutional review board, cancer patients being treated by our Oncology Department and who died while admitted in the hospital between January 1, 2010 and January 1, 2012 were identified. Patients who were under the age of 18 years, those with a diagnosis of leukemia and multiple myeloma were excluded. Also excluded were patients who did not receive any chemotherapy or other cancer treatment during the course of their illness. The inpatient, out-patient, paper and electronic medical records of the eligible patients were then reviewed to obtain demographic data, details of their malignancy and treatment, date, type and line of last chemotherapy, date of death, primary team managing them at time of death, whether they were being followed by our palliative care team, and whether they were on fixed dose opioids at time of death or not.
Statistical analysis
We chose TLCD cutoff at 60 days to divide the subjects into two cohorts, as 60 days was the most approximate period (rounded in 30 day intervals) to the median TCLD. Patient demographic characteristics, Eastern Cooperative Group performance status (ECOG-PS), type and line of last chemotherapy, primary team at time of death and proportion of patients with palliative care involvement were compared between the two cohorts using Chi-square test, with P < 0.05 considered as significant. Univariate analysis to determine significant association with risk factors to TLCD less than 60 days was performed using Mantel-Haenszel common odds ratio estimate. The factors found to a significant correlation in univariate analysis were then tested for independent significance by multivariate analysis using backward stepwise binary logistic regression method. For backward stepwise selection, significance levels of 0.05 and 0.15 were used for entry and removal respectively. Survival analyses were performed using Kaplan-Meier method and comparison of median survival between cohorts was performed using log-rank test. All statistical analyses were performed using IBM® SPSS® Statistics software version 20, Release 20.0.0, License Number ANTLR 2.7.5.
RESULTS
A total of 115 patient deaths meeting the above criteria were identified. The mean TLCD was 169.3 days, ranging from 1 to 1595 days. Eleven patients (9.56%) received chemotherapy during the last 2 weeks of life. Ten of these patients (91%) died due to cancer related complications (as opposed to chemotherapy toxicity). 41 patients (35.6% of total) received chemotherapy within the last 60 days of life, with 12 of them (29.3%) receiving chemotherapy during their last admission to the hospital. Majority of these 12 patients (83%) died from cancer related complications, as opposed to complications of chemotherapy. Diagnoses of patients in the two groups (divided by TLCD of 60 days) are shown in Figure 1.
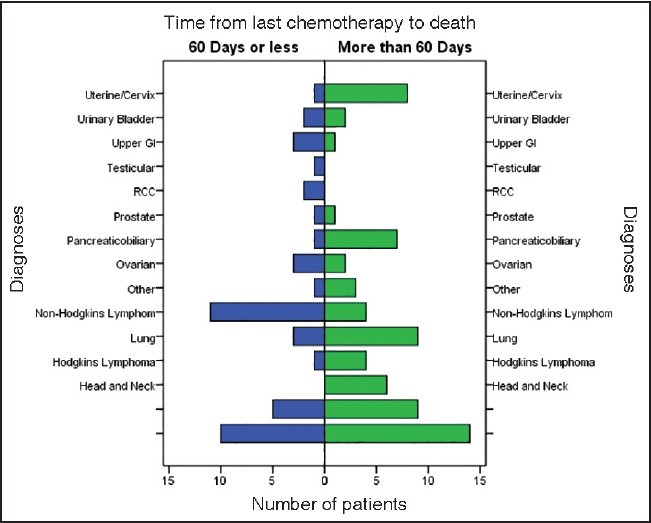
| Fig. 1 Distribution of patients according to diagnoses and time from last chemotherapy to death error bars: 95% confidence interval
Patient characteristics are shown in Table 1. A statistically similar proportion of patients received chemotherapy within or prior to the last 60 days of life, when compared according to gender, age and line of last treatment. In the cohort of patients who received chemotherapy in the last 60 days of life, there was a significant majority of patients who had ECOG-PS 3 or less, who died in the Intensive Care Unit (ICU), who died of chemotherapy related complications, who died under nonpalliative care service, who were not on fixed dose opioids and who received intravenous chemotherapy as opposed to oral (according to Chi-square test).
Table 1
Patient characteristics divided into two groups: TLCD of 60 or less days, and TLCD more than 60 days
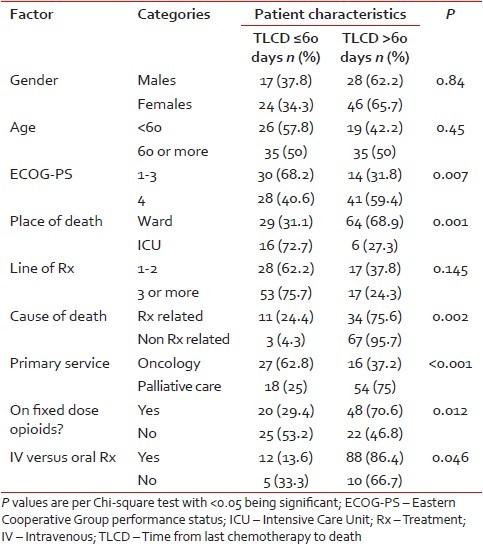
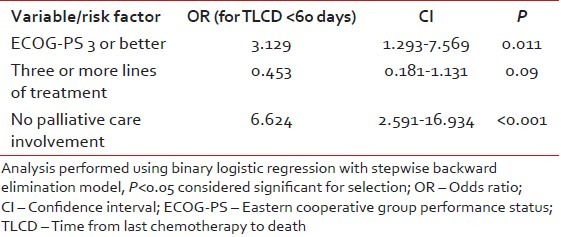
Table 2 shows risk factors for treatment with chemotherapy within 60 days of death [Table 3] according to univariate analysis. Patients with ECOG-PS 3 or better, those who died on nonpalliative care service, and those without any palliative care involvement had at least 2 fold likelihood of receiving chemotherapy within 60 days of death. Patients who received chemotherapy during the last 60 days of life were also 2.3 times as likely to die with complications of chemotherapy and to die in the ICU. Results of multivariate analysis showed that absence of involvement of palliative care team and ECOG-PS of 3 or better independently predisposed patients to receive chemotherapy within 60 days of death.
Table 2
Univariate analysis of patient variables predisposing them to receive chemotherapy within 60 days of death
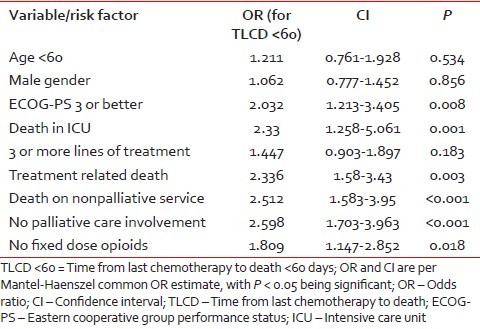
Table 3
Multivariate analysis of significant factors correlating with TLCP < 60 days
Figures 2 and 3 show Kaplan-Meier survival curves for patients after receiving their last chemotherapy. Patients who died under palliative care service had longer median survival (120 days) after last chemotherapy as compared to other patients [120 and 43 days respectively, P < 0.001, Figure 2]. Furthermore, patients had a longer median survival after last chemotherapy if there was any involvement of the palliative care team [116 and 35 days respectively, P < 0.001, Figure 3].
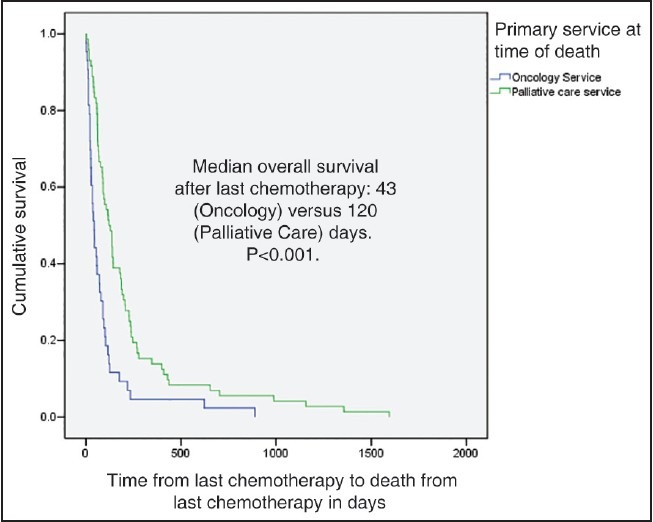
| Fig. 2 Kaplan-Meier graph showing survival in patients who died under oncology and palliative care services
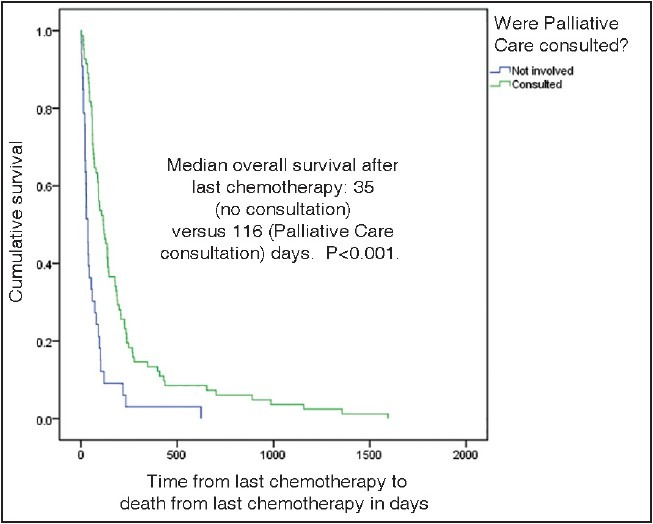
| Fig. 3 Kaplan-Meier graph showing survival difference in patients who had no palliative care consultation versus those who had palliative care consultation
DISCUSSION
Time from last chemotherapy to death is emerging as an important healthcare benchmark for improvement of quality of cancer care. [9,10] In a Canadian study, treatment with chemotherapy in the last 2 weeks of life correlated with poor EoL care. [11] However, there is evidence of an increasing trend of this practice over the last two decades, both in Canada [12] and the US. [13,14] In a Swedish study, 24% of cancer patients received chemotherapy in the last month of life, and this was related to unfavorable EoL outcomes. [15] In our study, the proportion of patients who received chemotherapy in the last 2 weeks of life was less (10%) as compared to 18.5-33.8% in Western studies, [13,16] however our study looked at only inpatient deaths so the comparison may not be accurate. Almost all of our patients who received chemotherapy within 2 weeks of death died of cancer related complications, which reflects the adverse quality of care association as seen in Western studies as discussed above.
Various studies have looked at factors associated with administration of chemotherapy near death. [5,6,17,18,19] Our univariate analysis indicates that in our population, patients with better performance status and those receiving intravenous chemotherapy (as opposed to oral) had higher incidence of treatment near death. To our knowledge, previous studies have not reported this association. These findings suggest that performance status may not be the only determinant of a favorable outcome from chemotherapy. Furthermore, more aggressive treatment (with IV chemotherapy as opposed to oral) may not lead to improved disease outcome. Our results also suggested that patients who received chemotherapy within 60 days of death were more likely to die in the ICU, and to die as a complication of chemotherapy, which are both considered adverse indicators of EoL care. [11,20]
Involvement of palliative care services has been shown in various studies to improve quality of EoL care of cancer patients. [21,22,23] Our results indicate that patients who had palliative care involvement or who died under the palliative care service were less likely to have received chemotherapy near death, and more likely to have been on schedule opioids at time of death [Tables [Tables11 and and2],2], both of these factors reflecting good palliation at the EoL.
Prior studies particularly in NSCLC have indicated that chemotherapy near death does not lead to improved survival of patients. [21,24] Available data also suggests that earlier involvement of palliative care, [21] or having information regarding hospice care [17] can lead to improved survival from last chemotherapy to death. Interestingly, our results mirror these previous findings, showing improvement in median overall survival from last chemotherapy to death in patients dying under palliative care service [Figure 2] and in patients who were being followed by our palliative care team [Figure 3]. To the best of our knowledge, this is the first data from the Middle Eastern region showing this survival difference relative to timing of chemotherapy and palliative care involvement consistent with Western data. These findings reiterate the importance of earlier involvement of palliative care in cancer patients, regardless of regional and cultural variations.
A limitation in our study was unavailability of outpatient mortality data, as we looked at only inpatient deaths. This may have led to a degree of selection bias as a substantial number of patients may have been admitted due to complications of chemotherapy and died from treatment toxicity. However, such patients constitute only a minority (39.1%) of all our cases, with the remainder of mortalities being from cancer related causes. Therefore, we believe our study sample was well balanced and representative of the overall cancer mortality pattern in the region. Another limitation of our study was a limited sample size for survival analysis.
CONCLUSIONS
Time from last chemotherapy to death is emerging as a quality of care endpoint in cancer patients. Our data from Middle Eastern cancer patients confirms previous findings from Western studies that earlier involvement of palliative care teams leads to better EoL care for cancer patients. Aggressive treatment with chemotherapy near the EoL does not necessarily lead to improved survival of these patients, and may lead to worsening of EoL care outcomes.
ACKNOWLEDGMENTS
Mohammad Iqbal Ansari (for providing valuable ideas and guidance).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Distribution of patients according to diagnoses and time from last chemotherapy to death error bars: 95% confidence interval

| Fig. 2 Kaplan-Meier graph showing survival in patients who died under oncology and palliative care services

| Fig. 3 Kaplan-Meier graph showing survival difference in patients who had no palliative care consultation versus those who had palliative care consultation


 PDF
PDF  Views
Views  Share
Share

