Toxicity Profile of Double-agent Adjuvant Chemotherapy after Concurrent Chemoradiation and Brachytherapy in Locally Advanced Cervical Cancer: Comparison with Standard Chemoradiation Protocol
CC BY-NC-ND 4.0 Indian J Med Paediatr Oncol 2019; 40(S 01): S6-S12
DOI: DOI: 10.4103/ijmpo.ijmpo_171_17
Abstract
Introduction: Carcinoma cervix is the most common gynecological malignancy in India and a major cause of cancer mortality and morbidity in the females despite Concurrent chemoradiotherapy (CCRT). Attempts are on to improved overall survival by addition of adjuvant chemotherapy (ACT) to CCRT. Aim: The aim of this study is to establish toxicity profile of double-agent ACT after CCRT and ICRT in locally advanced cervical cancer (LACC) and to compare it with standard chemoradiation protocol. Materials and Methods: Patients were randomized into two arms: in conventional arm (Arm 1, n = 23), patients received a standard protocol of weekly injection cisplatin 40 mg/m2 concurrently with pelvic external beam radiotherapy (5040cGy/28 fractions) followed by ICRT (03 fractions of 7 Gy each). In interventional arm (Arm 2, n = 24), patients received CCRT/ICRT protocol; and were further offered ACT with three cycles of consolidation chemotherapy using injection paclitaxel and injection carboplatin every 3 weeks after CCRT and ICRT. Results: The incidence of anemia was 14/23 (50% Grade 1) in Arm 1 and 12/24 in Arm 2 (17% Grade 1, rest higher grade). In Arm 2, 37% of patients had Grade 2 neuropathy and 16% of patients had Grade 1 alopecia, whereas nil incidence was reported in Arm 1 (P = 0.005 and 0.04, respectively). Grade 3 neutropenia was observed in 4/23 (17%) patients of Arm 1 and 8/24 patients (33%) of Arm 2. None of the patients in Arm 1 required indoor supportive care while 4/24 patients (17%) were managed as an indoor patient. Among late toxicities, in Arm 2, the incidence of Grade 2 and Grade 3 anemia was 42%, whereas in Arm 1, its incidence was 22%. In Arm 1, no patient exhibited features of neuropathy, whereas, in Arm 2, 12/24 (50%) of the patients had neuropathy (P value for these two late events was <0 class="b" xss=removed>Conclusion: Exhibition of ACT with injection Paclitaxel and injection carboplatin in locally advanced carcinoma cervix is a technically viable option with manageable toxicity.
Keywords
Acute toxicities - adjuvant chemotherapy - concurrent chemoradiation therapy - delayed toxicities - locally advanced cancer cervixPublication History
Publication Date:
24 May 2021 (online)
2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:?Carcinoma cervix is the most common gynecological malignancy in India and a major cause of cancer mortality and morbidity in the females despite Concurrent chemoradiotherapy (CCRT). Attempts are on to improved overall survival by addition of adjuvant chemotherapy (ACT) to CCRT.?Aim:?The aim of this study is to establish toxicity profile of double-agent ACT after CCRT and ICRT in locally advanced cervical cancer (LACC) and to compare it with standard chemoradiation protocol.?Materials and Methods:?Patients were randomized into two arms: in conventional arm (Arm 1,?n?= 23), patients received a standard protocol of weekly injection cisplatin 40 mg/m2?concurrently with pelvic external beam radiotherapy (5040cGy/28 fractions) followed by ICRT (03 fractions of 7 Gy each). In interventional arm (Arm 2,?n?= 24), patients received CCRT/ICRT protocol; and were further offered ACT with three cycles of consolidation chemotherapy using injection paclitaxel and injection carboplatin every 3 weeks after CCRT and ICRT.?Results:?The incidence of anemia was 14/23 (50% Grade 1) in Arm 1 and 12/24 in Arm 2 (17% Grade 1, rest higher grade). In Arm 2, 37% of patients had ?Grade 2 neuropathy and 16% of patients had Grade 1 alopecia, whereas nil incidence was reported in Arm 1 (P?= 0.005 and 0.04, respectively). Grade 3 neutropenia was observed in 4/23 (17%) patients of Arm 1 and 8/24 patients (33%) of Arm 2. None of the patients in Arm 1 required indoor supportive care while 4/24 patients (17%) were managed as an indoor patient. Among late toxicities, in Arm 2, the incidence of Grade 2 and Grade 3 anemia was 42%, whereas in Arm 1, its incidence was 22%. In Arm 1, no patient exhibited features of neuropathy, whereas, in Arm 2, 12/24 (50%) of the patients had neuropathy (P?value for these two late events was <0 class="b" xss=removed>Conclusion:?Exhibition of ACT with injection Paclitaxel and injection carboplatin in locally advanced carcinoma cervix is a technically viable option with manageable toxicity.
Keywords
Acute toxicities - adjuvant chemotherapy - concurrent chemoradiation therapy - delayed toxicities - locally advanced cancer cervixIntroduction
Cervical carcinoma is the second most frequent cancer among women worldwide and most common gynecological cancer in Indian women.[1] [2] [3] It is the fourth leading cause of global cancer death among women with an estimated 528,000 new cases (accounting for around 12% of all cancers) and 266,000 deaths in 2012.[4] In India, 122,844 women are annually diagnosed with cervical cancer and 67,477 die from the disease. It is estimated that cervical cancer will occur in approximately 1 in 53 Indian women during their lifetime compared with 1 in 100 women in more developed regions of the world.[5] [6] [7] [8] In addition to a higher incidence of cervical cancer in less developed regions, patients in these areas have a higher proportion of locally advanced stages, including stage IIB to IVA of the International Federation of Gynecology and Obstetrics (FIGO) staging classification, or advanced stage IVB cancers.[9] Overall, 80%?90% of patients present with advanced stage with the bulky central disease.[5]
Since 1999, the mainstay of treatment for locally advanced cervical cancer (LACC) has been injectio cisplatin-based concurrent chemoradiation therapy (CCRT), following a National Cancer Institute clinical announcement.[10] Radiotherapy (RT) and concurrent chemotherapy were shown to improve the control of pelvic disease and significantly increased overall survival (OS) rates in various randomized trials and is considered as the standard of care for patients with bulky stage IB disease, stage IIB through IVA and high-risk cervical cancer cases.[11] [12] However, outcomes in this disease remained suboptimal, with long-term progression-free survival (PFS) and OS rates of approximately 60%.[1] [13] [14] Local and distant failures of 17% and 18%, respectively, in LACC after CCRT were still encountered.[15] Interventions provided to improve treatment outcomes include chemotherapy administered before CCRT (neoadjuvant chemotherapy [NACT]) and additional chemotherapy given after the standard treatment, which is referred to as ?consolidation chemotherapy? or ?ACT?.[16] [17] [18]
The objective of ACT after completion of RT or CCRT is to eradicate the residual disease in the pelvis and treating occult disease outside the pelvic radiation field.[1] The results of the ACT after CCRT in LACC have shown superiority over CCRT alone, but most of these data are from western literature with some disparity.[18] [19] [20] OS rates >80% to 90?hieved with CCRT followed by the ACT were higher than the 60% to 65% rates obtained with CCRT alone.[21] As chemotherapy can cause toxicities, potential survival advantages should outweigh these disadvantages. Due to paucity of data on safety and tolerance of ACT in LACC in Indian scenario, a prospective study was conducted to study the toxicity profile of double-agent ACT after CCRT and intracavitary RT (ICRT) in LACC and to compare it with standard chemoradiation protocol.
Materials and Methods
Study design
This is an experimental prospective randomized study; comparative and interventional in nature; that was carried out at the Oncology Center of a tertiary care super-specialty hospital of government setup in a developing country over 2 years duration.
Sample size
The following formula was used to calculate the required number of patients in the study:?N?=4 PQ/L2; Where?P?is the prevalence of cervical cancer, which is 10% approximately at this Hospital;?Q?is 100-P and?L?= Permissible Error = 10%. Hence,?n?= 4 ? 10 ? 90/100 = 36 (approximated to 40). Thus, at least 40 patients needed to be enrolled, 20 in each arm.
Inclusion criteria
The inclusion criteria were histologically confirmed squamous cell carcinoma/adenocarcinoma cervix, stage IIB to IVB (limited to paraaortic lymph node involvement without any distant metastasis), no previous malignancy/radiation to pelvis/chemotherapy, age group <70>
Treatment protocol
There were two arms of the study, Conventional Arm (Arm 1) and Interventional arm (Arm 2) having 23 and 24 patients, respectively. The patients were randomized by simple random sampling technique (Chit-Pull system). In Arm 1, patients were managed by the standard protocol of weekly injection cisplatin 40 mg/m 2 concurrently with pelvic RT, followed by ICRT. In Arm 2, after this standard CCRT and ICRT protocol, patients were further offered ACT with 3 cycles of ACT using injection paclitaxel (155 mg/m2) and injection carboplatin (AUC 5.0) every 3 weekly. In both arms, patients received pelvic RT 5040cGy/28 fractions @180cGy per fraction over 5?6 weeks; and in patients with evidence of paraaortic lymph node involvement on imaging, a paraaortic field was added to a dose of 4500cGy/25 fractions. This external beam radiotherapy was followed by ICRT with high dose rate brachytherapy (BCT) in the form of 03 fractions of 7 Gy each. During treatment and up to 3 months posttreatment, the patients were monitored for therapy-induced acute toxicities and were reviewed thereafter every 12 weekly for delayed toxicities.
Results
Patient-related characteristics
The mean age of the patients was 56.4 years (range: 39?69 years) and 52.7 years (range: 33?70 years) in Arms 1 and 2, respectively. The age-wise distribution of the 2 arms is summarized in [Table 1]. Maximum incidence of the disease was seen in the age group of 61?70 years in the conventional arm while 41?50 years in the interventional arm. 6/23 patients in each Arm (26% and 25% in Arms 1 and 2, respectively) had comorbidities such as hypertension, diabetes mellitus, and hypothyroidism. The KPS of patients in both arms were 90% for all the patients at the time of pretreatment evaluation excluding three patients in Arm 1 and one patient in Arm 2 with KPS of 80%. The average pretreatment hemoglobin in Arm 1 was 11.04 g/dl and in Arm 2 was 11.13 g/dl.
|
Age group (years) |
Arm 1 |
Arm 2 |
Total |
? |
P |
|---|---|---|---|---|---|
|
Arm 1 ? Conventional arm (n=23); Arm 2 ? Interventional arm (n=24) |
|||||
|
<40> |
1 |
2 |
3 |
2.062 |
0.56 |
|
41-50 |
7 |
10 |
17 |
||
|
51-60 |
6 |
7 |
13 |
||
|
61-70 |
9 |
5 |
14 |
||
|
Total |
23 |
24 |
47 |
||
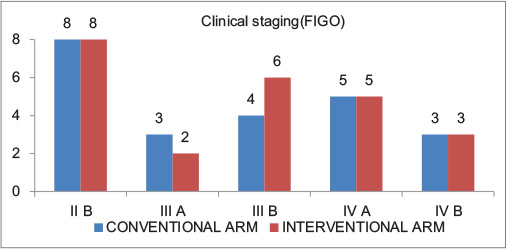
|?Figure. 1? Clinical Stages in Arm 1 (Conventional Arm, n = 23); and Arm 2 (Interventional arm, n = 24)|
|
Arm 1 |
Arm 2 |
Total |
Pearson ?2 |
P |
|
|---|---|---|---|---|---|
|
Arm 1 ? Conventional arm (n=23); Arm 2 ? Interventional arm (n=24); LNs ? Lymph nodes |
|||||
|
Pelvic LNs |
|||||
|
No |
3 |
5 |
8 |
0.505 |
0.477 |
|
Yes |
20 |
19 |
39 |
||
|
Inguinal LNs |
|||||
|
No |
23 |
23 |
46 |
0.979 |
0.322 |
|
Yes |
0 |
1 |
1 |
||
|
Para-aortic LNs |
|||||
|
No |
20 |
21 |
41 |
0.003 |
0.955 |
|
Yes |
3 |
3 |
6 |
||
|
Toxicity |
Grade |
Arm 1 |
Arm 2 |
Total |
Pearson ?2 |
P |
|---|---|---|---|---|---|---|
|
Arm 1 ? Conventional arm (n=23); Arm 2 ? Interventional arm (n=24); AKI ? Acute kidney injury |
||||||
|
Nausea/vomiting |
0 |
15 |
19 |
34 |
1.268 |
0.53 |
|
1 |
1 |
1 |
2 |
|||
|
2 |
7 |
4 |
11 |
|||
|
AKI |
0 |
19 |
23 |
42 |
2.361 |
0.307 |
|
1 |
3 |
1 |
4 |
|||
|
3 |
1 |
0 |
1 |
|||
|
Anaemia |
0 |
9 |
12 |
21 |
4.93 |
0.177 |
|
1 |
7 |
2 |
9 |
|||
|
2 |
3 |
7 |
10 |
|||
|
3 |
4 |
3 |
7 |
|||
|
Neutropenia |
0 |
19 |
16 |
35 |
1.57 |
0.21 |
|
3 |
4 |
8 |
12 |
|||
|
Skin reaction |
0 |
20 |
19 |
39 |
4.673 |
0.097 |
|
1 |
2 |
0 |
2 |
|||
|
2 |
1 |
5 |
6 |
|||
|
Neuropathy |
0 |
23 |
15 |
38 |
10.668 |
0.005 |
|
2 |
0 |
7 |
7 |
|||
|
3 |
0 |
2 |
2 |
|||
|
Cystitis |
0 |
19 |
18 |
37 |
2.007 |
0.367 |
|
2 |
3 |
6 |
9 |
|||
|
4 |
1 |
0 |
1 |
|||
|
Proctitis |
0 |
21 |
17 |
38 |
5.902 |
0.052 |
|
2 |
1 |
7 |
8 |
|||
|
4 |
1 |
0 |
1 |
|||
|
Enteritis |
0 |
21 |
16 |
37 |
1.139 |
0.286 |
|
2 |
2 |
4 |
6 |
|||
|
Vaginal stricture |
0 |
21 |
20 |
41 |
2.004 |
0.367 |
|
1 |
2 |
2 |
4 |
|||
|
2 |
0 |
2 |
2 |
|||
|
Alopecia |
0 |
23 |
20 |
43 |
4.19 |
0.041 |
|
1 |
0 |
4 |
4 |
|||
|
Total |
23 |
24 |
47 |
|||
|
Toxicity |
Grade |
Arm 1 |
Arm 2 |
Total |
Pearson ?2 |
P |
|---|---|---|---|---|---|---|
|
Arm 1 ? Conventional arm (n=23); Arm 2 ? Interventional arm (n=24) |
||||||
|
Anaemia |
0 |
10 |
14 |
24 |
14.106 |
0.003 |
|
1 |
8 |
0 |
8 |
|||
|
2 |
2 |
9 |
11 |
|||
|
3 |
3 |
1 |
4 |
|||
|
Neutropenia |
0 |
23 |
24 |
47 |
||
|
Neuropathy |
0 |
23 |
12 |
35 |
15.443 |
0.001 |
|
1 |
0 |
1 |
1 |
|||
|
2 |
0 |
10 |
10 |
|||
|
3 |
0 |
1 |
1 |
|||
|
Cystitis |
0 |
21 |
21 |
42 |
0.179 |
0.672 |
|
2 |
2 |
3 |
5 |
|||
|
Proctitis |
0 |
20 |
19 |
39 |
2.291 |
0.318 |
|
2 |
2 |
5 |
7 |
|||
|
3 |
1 |
0 |
1 |
|||
|
Vaginal stricture |
0 |
13 |
19 |
32 |
4.217 |
0.239 |
|
1 |
2 |
0 |
2 |
|||
|
2 |
5 |
4 |
9 |
|||
|
3 |
3 |
1 |
4 |
|||
|
Alopecia |
0 |
23 |
20 |
43 |
4.19 |
0.123 |
|
1 |
0 |
3 |
3 |
|||
|
2 |
0 |
1 |
1 |
|||
|
Total |
- |
23 |
24 |
47 |
- |
- |
- Tangjitgamol S, Katanyoo K, Laopaiboon M, Lumbiganon P, Manusirivithaya S, Supawattanabodee B. et al.?Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst Rev 2014; 12:?CD010401
- Varghese SS, Ram TS, Pavamani SP, Thomas EM, Jeyaseelan V, Viswanathan PN. et al.?Concurrent chemo-irradiation with weekly cisplatin and paclitaxel in the treatment of locally advanced squamous cell carcinoma of cervix: A phase II study. J Cancer Res Ther 2014; 10: 330-6
- Sagae S, Monk BJ, Pujade-Lauraine E, Gaffney DK, Narayan K, Ryu SY. et al.?Advances and concepts in cervical cancer trials: A Road map for the future. Int J Gynecol Cancer 2016; 26: 199-207
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. et al.?GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Last accessed on 2017 May 12].
- <a

|?Figure. 1? Clinical Stages in Arm 1 (Conventional Arm, n = 23); and Arm 2 (Interventional arm, n = 24)|
References
- Tangjitgamol S, Katanyoo K, Laopaiboon M, Lumbiganon P, Manusirivithaya S, Supawattanabodee B. et al. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst Rev 2014; 12: CD010401
- Varghese SS, Ram TS, Pavamani SP, Thomas EM, Jeyaseelan V, Viswanathan PN. et al. Concurrent chemo-irradiation with weekly cisplatin and paclitaxel in the treatment of locally advanced squamous cell carcinoma of cervix: A phase II study. J Cancer Res Ther 2014; 10: 330-6
- Sagae S, Monk BJ, Pujade-Lauraine E, Gaffney DK, Narayan K, Ryu SY. et al. Advances and concepts in cervical cancer trials: A Road map for the future. Int J Gynecol Cancer 2016; 26: 199-207
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Last accessed on 2017 May 12].
- Singh JK, Chauhan R. Management of locally advanced cancer cervix an Indian perspective. Rev Recent Clin Trials 2015; 10: 298-301
- Bobdey S, Sathwara J, Jain A, Balasubramaniam G. Burden of cervical cancer and role of screening in India. Indian J Med Paediatr Oncol 2016; 37: 278-85
- Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health 2015; 7: 405-14
- Asano H, Todo Y, Watari H. Adjuvant chemotherapy for early-stage cervical cancer. Chin J Cancer Res 2016; 28: 228-34
- Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J. et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J Clin Oncol 2004; 22: 3113-9
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of cheoradiotherapy for cervical cancer: Individual patient data meta-analysis. Cochrane Database Syst Rev 2010; 1: CD008285 DOI: 10.1002/14651858.CD008285.
- Mitra D, Ghosh B, Kar A, Basu S, Deb AR, Sur PK. et al. Role of chemoradiotherapy in advanced carcinoma cervix. J Indian Med Assoc 2006; 104: 432, 434, 436
- Zarb JJ, Verma J, Monk BJ, Wolfson AH. New strategies for multimodality therapy in treating locally advanced cervix cancer. Semin Radiat Oncol 2016; 26: 344-8
- Jelavi TB, Mi e BP, Strikic A, Ban M, Vrdoljak E. Adjuvant chemotherapy in locally advanced cervical cancer after treatment with concomitant chemoradiotherapy Room for improvement . Anticancer Res 2015; 35: 4161-5
- Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L. et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 2005; 3: CD002225
- Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J. et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004; 22: 872-80
- Singh U, Ahirwar N, Rani AK, Singh N, Sankhwar P, Qureshi S. et al. The efficacy and safety of neoadjuvant chemotherapy in treatment of locally advanced carcinoma cervix. J Obstet Gynaecol India 2013; 63: 273-8
- Vrdoljak E, Omrcen T, Novakovi ZS, Jelavi TB, Prskalo T, Hrepi D. et al. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy for women with locally advanced carcinoma of the uterine cervix Final results of a prospective phase II-study. Gynecol Oncol 2006; 103: 494-9
- Zhang MQ, Liu SP, Wang XE. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: Preliminary results of a phase II study. Int J Radiat Oncol Biol Phys 2010; 78: 821-7
- Choi IJ, Cha MS, Park ES, Han MS, Choi Y, Je GH. et al. The efficacy of concurrent cisplatin and 5-flurouracil chemotherapy and radiation therapy for locally advanced cancer of the uterine cervix. J Gynecol Oncol 2008; 19: 129-34
- Domingo E, Lorvidhaya V, de Los Reyes R, Syortin T, Kamnerdsupaphon P, Lertbutsayanukul C. et al. Capecitabine-based chemoradiotherapy with adjuvant capecitabine for locally advanced squamous carcinoma of the uterine cervix: Phase II results. Oncologist 2009; 14: 828-34
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008; 26: 5802-12
- Ali N, Valimohammad AT, Abbasi AN, Mansha MA, Asim Hafiz A, Qureshi BM. Chemoradiation and the role of adjuvant chemotherapy in lymph nodal Metastatic cervical cancer. J Glob Oncol 2017; Published online on jgo.org on 2017:1-4. Available from:https://doi.org/10.1200/JGO.2017.009852. [Last accessed on 2017 May 12].
- Abe A, Furumoto H, Nishimura M, Irahara M, Ikushima H. Adjuvant chemotherapy following concurrent chemoradiotherapy for uterine cervical cancer with lymphadenopathy. Oncol Lett 2012; 3: 571-6
- Tang J, Tang Y, Yang J, Huang S. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol 2012; 125: 297-302
- Due as-Gonz lez A, Zarb JJ, Patel F, Alcedo JC, Beslija S, Casanova L. et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011; 29: 1678-85
- Lorvidhaya V, Chitapanarux I, Sangruchi S, Lertsanguansinchai P, Kongthanarat Y, Tangkaratt S. et al. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: A randomized trial. Int J Radiat Oncol Biol Phys 2003; 55: 1226-32
- Sangkittipaiboon S. Long-term outcomes of concurrent chemoradiotherapy with weekly carboplatin in locally-advanced carcinoma of the uterine cervix patients. J Med Assoc Thai 2014; 97: 12-9
- McCormack M, Kadalayil L, Hackshaw A, Hall-Craggs MA, Symonds RP, Warwick V. et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. Br J Cancer 2013; 108: 2464-9


 PDF
PDF  Views
Views  Share
Share

