Treatment Challenges and Survival Analysis of Human Epidermal Growth Factor Receptor 2-positive Breast Cancer in Real World
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 22-27
DOI: DOI: 10.4103/0971-5851.203511
Abstract
Context: Advent of trastuzumab has brought tremendous changes in the survival of human epidermal growth factor receptor 2 (Her2)-positive breast cancer patients. Despite the availability of the drug, it is still out of reach for many patients. There is very limited real world data regarding treatment challenges and survival analysis of these patients. Aims and Objectives: Primary objective is disease-free survival (DFS) and secondary objective is overall survival (OS) and toxicity profile. Statistics: Statistical analysis is done using GraphPad Prism 7.02. Materials and Methods: This is a retrospective study of all patients diagnosed with Her2-positive (Her2+) nonmetastatic invasive breast cancer from January 2007 to December 2013. Results: In the period of this study, 885 patients are diagnosed with carcinoma breast, of which 212 are Her2/neu positive (23.9%). Of the 212 patients, only 76 (35.8%) patients received trastuzumab along with chemotherapy. Patients receiving trastuzumab with chemotherapy have longer 5-year DFS compared to those receiving chemotherapy alone, 92% and 52.6%, respectively (P = 0.0001). Five-year OS is 90.5% and 41.7% in those patients who received chemotherapy with and without trastuzumab, respectively (P = 0.0001). Seven patients (9.45%) developed Grade II reversible diastolic dysfunction. Grade II/III peripheral neuropathy due to paclitaxel is the main adverse effect seen in 21 patients. Conclusion: In spite of improvement in DFS and OS with trastuzumab, the number of patient receiving targeted therapy is very low due to financial constraints which need to be addressed to bridge the gap in survival of Her2+ patients.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Advent of trastuzumab has brought tremendous changes in the survival of human epidermal growth factor receptor 2 (Her2)-positive breast cancer patients. Despite the availability of the drug, it is still out of reach for many patients. There is very limited real world data regarding treatment challenges and survival analysis of these patients.
Aims and Objectives:
Primary objective is disease-free survival (DFS) and secondary objective is overall survival (OS) and toxicity profile.
Statistics:
Statistical analysis is done using GraphPad Prism 7.02.
Materials and Methods:
This is a retrospective study of all patients diagnosed with Her2-positive (Her2+) nonmetastatic invasive breast cancer from January 2007 to December 2013.
Results:
In the period of this study, 885 patients are diagnosed with carcinoma breast, of which 212 are Her2/neu positive (23.9%). Of the 212 patients, only 76 (35.8%) patients received trastuzumab along with chemotherapy. Patients receiving trastuzumab with chemotherapy have longer 5-year DFS compared to those receiving chemotherapy alone, 92% and 52.6%, respectively (P = 0.0001). Five-year OS is 90.5% and 41.7% in those patients who received chemotherapy with and without trastuzumab, respectively (P = 0.0001). Seven patients (9.45%) developed Grade II reversible diastolic dysfunction. Grade II/III peripheral neuropathy due to paclitaxel is the main adverse effect seen in 21 patients.
Conclusion:
In spite of improvement in DFS and OS with trastuzumab, the number of patient receiving targeted therapy is very low due to financial constraints which need to be addressed to bridge the gap in survival of Her2+ patients.
Introduction
Human epidermal growth factor receptor 2 (Her2) is a 185-kd glycoprotein with tyrosine kinase activity. Overexpression of Her2 in breast cancer is a key feature of pathobiology of the disease and is associated with poor prognosis.[1,2,3] Amplification is the primary mechanism of Her2 overexpression.[4] Approximately 20% of all newly diagnosed invasive breast carcinomas are Her2-positive (Her2+). The proportion is higher among tumors with higher grade and in patients with positive nodal status.
Trastuzumab is a humanized monoclonal antibody directed to the external domain of Her2. Four large randomized trials in early breast cancer have shown that treatment with trastuzumab can significantly improve the patient outcome when given alongside and/or subsequent to adjuvant chemotherapy.[5,6,7,8] Decrease in recurrence by approximately one-half and mortality by approximately one-third have been demonstrated with the use of trastuzumab. As a result, trastuzumab has become the standard of care for the treatment of Her2+ early breast cancer.
From the available data, the cost of treating Her2+ breast cancer in adjuvant setting in a developed country varies from US$ 70,000 to 80,000/patient while the Gross National Income per capita is
While India has access to highly skilled medical professionals and institutes that can provide the state-of-the-art medical care comparable to the best in the world, these are often limited to major cities and are inaccessible to the poor. India is ranked 135 in the world in terms of the human development index, which assesses the long-term progress in health and social well-being of a country.[9] Only 1%–2% of the country's gross domestic product (GDP) goes toward health and an estimated 55% of the population in India is poor by the multidimensional poverty index.[10] Approximately 39 million people are falling below poverty line every year.[11] It is estimated that 80% of medical expenses are met by out-of-pocket expenses, which means majority of them cannot afford expensive treatment. The government-run insurance schemes which are in vogue in some states in India do not provide for expensive treatments such as monoclonal antibodies. All these ultimately make the patients' abandon the expensive part of therapy, however, best the outcome might be.
Although significant advances have been made in the adjuvant treatment of Her2+ breast cancer, majority of them are based on well-controlled clinical trials from countries with universal health care. There is very limited real world data of the epidemiology and clinical outcome of Her2+ breast cancer patients.
Our study reflects the actual number of breast cancer patients with Her2 positivity undergoing treatment in our institute and their survival outcome compared to those not taking trastuzumab and treatment challenges.
Introduction
Human epidermal growth factor receptor 2 (Her2) is a 185-kd glycoprotein with tyrosine kinase activity. Overexpression of Her2 in breast cancer is a key feature of pathobiology of the disease and is associated with poor prognosis.[1,2,3] Amplification is the primary mechanism of Her2 overexpression.[4] Approximately 20% of all newly diagnosed invasive breast carcinomas are Her2-positive (Her2+). The proportion is higher among tumors with higher grade and in patients with positive nodal status.
Trastuzumab is a humanized monoclonal antibody directed to the external domain of Her2. Four large randomized trials in early breast cancer have shown that treatment with trastuzumab can significantly improve the patient outcome when given alongside and/or subsequent to adjuvant chemotherapy.[5,6,7,8] Decrease in recurrence by approximately one-half and mortality by approximately one-third have been demonstrated with the use of trastuzumab. As a result, trastuzumab has become the standard of care for the treatment of Her2+ early breast cancer.
From the available data, the cost of treating Her2+ breast cancer in adjuvant setting in a developed country varies from US$ 70,000 to 80,000/patient while the Gross National Income per capita is
While India has access to highly skilled medical professionals and institutes that can provide the state-of-the-art medical care comparable to the best in the world, these are often limited to major cities and are inaccessible to the poor. India is ranked 135 in the world in terms of the human development index, which assesses the long-term progress in health and social well-being of a country.[9] Only 1%–2% of the country's gross domestic product (GDP) goes toward health and an estimated 55% of the population in India is poor by the multidimensional poverty index.[10] Approximately 39 million people are falling below poverty line every year.[11] It is estimated that 80% of medical expenses are met by out-of-pocket expenses, which means majority of them cannot afford expensive treatment. The government-run insurance schemes which are in vogue in some states in India do not provide for expensive treatments such as monoclonal antibodies. All these ultimately make the patients' abandon the expensive part of therapy, however, best the outcome might be.
Although significant advances have been made in the adjuvant treatment of Her2+ breast cancer, majority of them are based on well-controlled clinical trials from countries with universal health care. There is very limited real world data of the epidemiology and clinical outcome of Her2+ breast cancer patients.
Our study reflects the actual number of breast cancer patients with Her2 positivity undergoing treatment in our institute and their survival outcome compared to those not taking trastuzumab and treatment challenges.
Results
In the 7-year study, 885 patients are diagnosed with carcinoma breast. Of these, 209 are Her2+ by IHC and 71 are Her2/neu equivocal. Receptor status of all patients is shown in Table 1. Of the 71 Her2 equivocal patients, only ten patients got FISH test done, out of which 3 are positive.
Table 1
Receptor expression in all patients

|
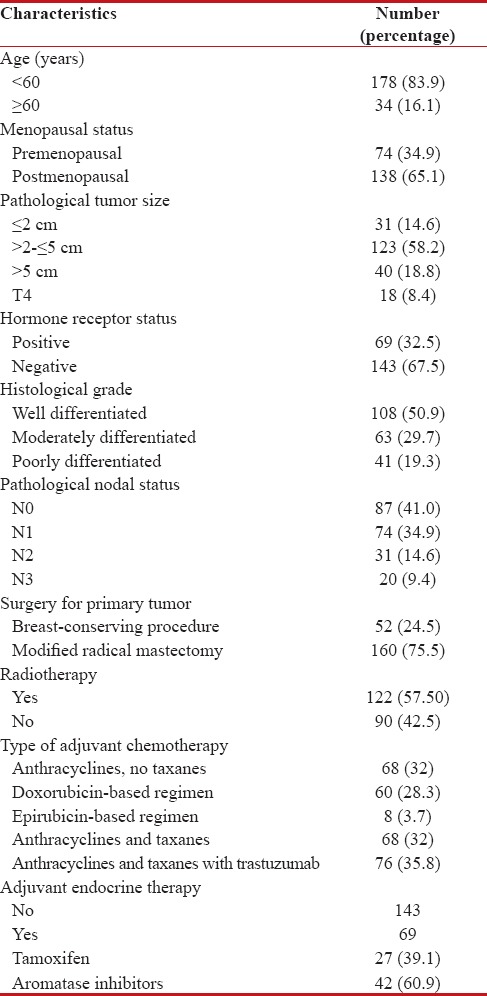
The median age is 50 years (range 27–76) in the study. Baseline demographic, clinicopathological, and treatment characteristics of all Her2+ patients are summarized in Table 2.
Table 2
Baseline demographic, clinicopathological, and treatment characteristics
|
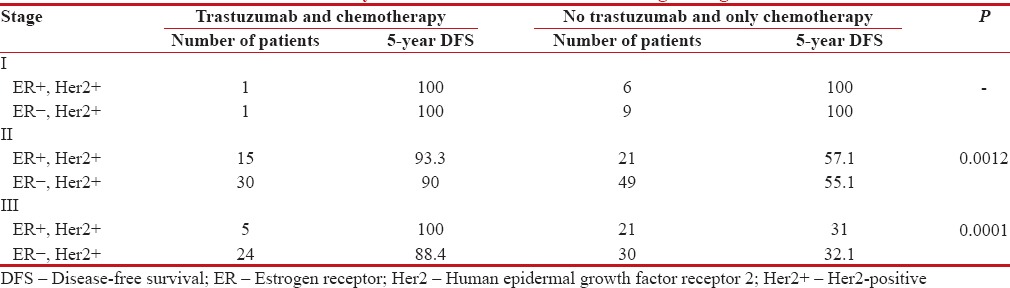
Of the 212 Her2+ patients, only 76 (35.8%) patients received trastuzumab along with chemotherapy. Of the 76, 54 patients (71.05%) received treatment under insurance scheme, 14 patients (18.4%) under clinical trial, and eight patients (10.5%) are self-paying which is depicted in Figure 1.
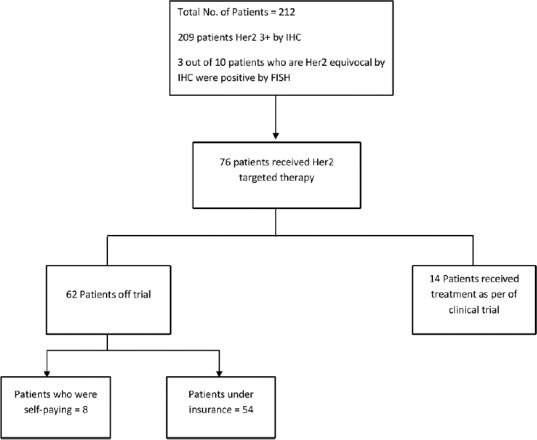
| Figure 3:Flow chart of treatment schema of all human epidermal growth factor receptor 2-positive patients
Chemotherapy is anthracycline-based in 97% of patients. Twenty-nine percent received taxane, 58% received radiotherapy. In patients who received trastuzumab along with chemotherapy, the 5-year DFS is 92% while it is 52.6% in patients who received chemotherapy alone (P = 0.0001) which is depicted in Figure 2. The 5-year DFS according to the receptor status and treatment received is shown in the Figures Figures3,3, ,44 and Table 3. Five-year OS is 90.5% and 41.7% in those patients who received chemotherapy with and without trastuzumab, respectively (P = 0.0001) which is shown in Figure 5.
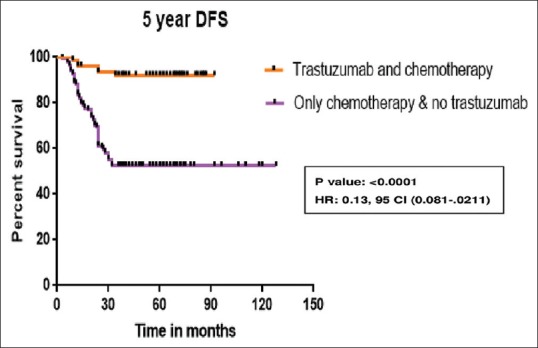
| Figure 2:Five-year disease-free survival of all human epidermal growth factor receptor 2-positive patients
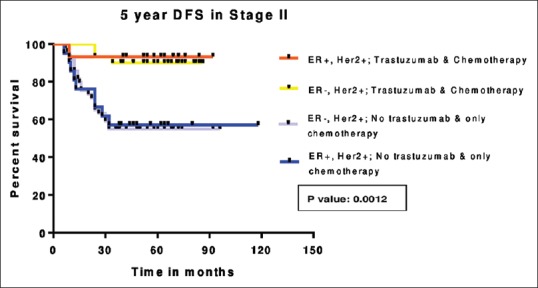
| Figure 3:Five-year disease-free survival in Stage II
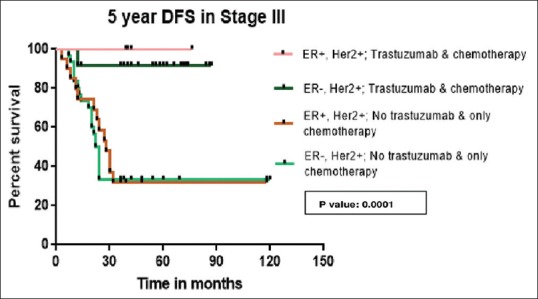
| Figure 4:Five-year disease-free survival in Stage III
Table 3
5-year disease-free survival according to stage
|
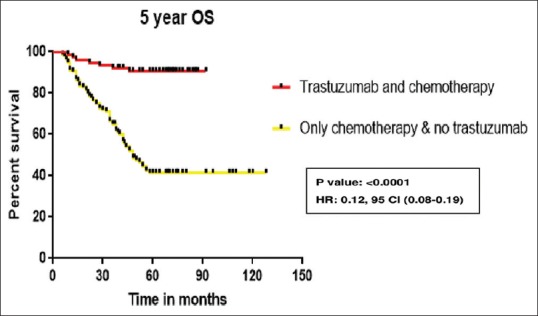
| Figure 5:Five-year overall survival of all human epidermal growth factor receptor 2-positive patients
Of the 76 patients who received trastuzumab, seven patients (9.2%) developed Grade II diastolic dysfunction requiring withholding of trastuzumab. Trastuzumab was restarted in five patients in 2 months. Grade II/III peripheral neuropathy due to paclitaxel is the main adverse effect seen in 21 patients.
Discussion
The incidence of breast cancer has increased globally over the last several decades;[13,14,15] with the greatest increase seen in Asian countries such as India, especially in premenopausal women.[16] It is estimated that the incidence has risen by 50%-between 1965 and 1985.[17] The rise in incidence of 0.5%–2%-per annum has been seen across all regions of India and in all age groups but more so in the younger age groups (<45>18] More than 80%-of Indian patients are younger than 60 years of age. With over 120,000 new breast cancer patients being diagnosed annually, it is the most common cancer among women in urban registries where it constitutes >30%-of all cancers in female and is the second most common cancer in women after cancer of the uterine cervix in the rural population-based cancer registries of Barshi.[19,20]
The median age of 50 years (range 27–76) in this cohort is strikingly different from that routinely reported in literature from developed countries where it is 61 years.[21] This lower median age in the present study could be due to a combination tertiary center referral bias and a different population pyramid structure in India and other developing economies, where the proportion of people over the age of 60 years is significantly lower. However, the possible role of additional genetic and environmental factors cannot be excluded.
Advent of trastuzumab has changed the management of breast cancer and the lives of many breast cancer patients worldwide. Although it was approved in adjuvant setting 10 years back, it is not affordable for most of the Indian patients. This single-center retrospective study over a 7-year period illustrates the challenges faced in managing Her2+ breast cancer patients in India. Most of the patients are not able to get the FISH test done if Her2 IHC is equivocal. This reflects challenge at diagnostic level itself.
Of the 212 patients who are diagnosed as positive for Her2, only 76 patients received trastuzumab which reflects the financial situation of the patients. If the patients included in the clinical trial are excluded, the total number of patients are even less.
Various studies show that the incidence of Her2 positivity in Indian population is between 26%-and 50%.[3,22,23] The incidence in our study is 23.9%. The exact incidence is yet to be known because a significant proportion of patients with Her2 equivocal are not undergoing further evaluation with FISH due to financial constraints. Significant heterogeneity between different hospitals with regard to diagnostic facilities available and access to trained personnel also contributes to it. In comparison with other studies from India, we found hormone receptor positivity in 41% of patients. This increase is mostly due to the increased and compulsory testing of almost all patients in the past few years.[24,25]
Coleman reported >80%-survival from breast cancer in North America and Europe compared with 60%-in middle-income countries and 40%-in low-income countries.[26] There is a significant difference in the survival rates in developed and developing countries mainly because of a lack of early detection programs and inadequate resources for treatment. A study by Gallagher CM et al. showed 5-year OS in patients receiving trastuzumab is 89%, similar to our study.[27] The 5-year OS of 43%-in patients treated with chemotherapy alone is similar to other Indian study by Jana et al.[28] There are no events relating to relapse after 3 years in our study indicating the aggressiveness and early relapse in patients with Her2+ breast cancer. There is very limited published data with trastuzumab from India, apart from clinical trials regarding the DFS and OS. Our study helps in contributing to literature.
The 9%-incidence of cardiotoxicity in our study is similar to the previous studies done by Russell et al. and Procter et al. and is reversible.[29,30] In five patients, rechallenge with the drug is done without any further complications. No cardiac-related mortality is seen. There is no increase in the incidence of CHF at a median follow-up of 3.3 years.
Different treatment centers in India have different costing structures based on whether they are private for-profit, private nonprofit, and fully or partly government-subsidized hospitals. In spite of these limitations, it is likely that these data are broadly representative of the experience of many tertiary centers in the country. The cost of treatment is around 1,000,000 ($16,000) with original trastuzumab. With many generics coming into market, the cost may be reduced by 25%. In a country with GDP per capita income of $1581, it is out of reach for most of the patients.[31]
From our data, it is clear to us that the single most important factor for not proceeding with treatment was lack of financial resources (90%) similar to other study from India by Ghosh et al.[32] Even though it is in the essential drug list,[33] it is not being provided at free of cost or subsidized price in most of the hospitals. It reiterates the need for incorporation of the drug into national and state health scheme programs. The government must note the importance of drugs which have a major impact on survival, especially in a cancer that is common among population and makes every effort to make it easily accessible.
Conclusion
Although the incidence of Her2 positivity is high, the number of patients actually receiving the targeted therapy is very low. Herculean efforts are need in terms of cost modification to bridge the gap in survival of Her2+ patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12.
- Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. ErbB-2 expression and its association with other biological parameters of breast cancer among Indian women. Indian J Cancer 2010;47:8-15.
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809-20.
- Malik K. United Nations Development Programme Report. Human Development Report; 2014. Available from: http://www.hdr.undp.org/en/content/humandevelopment-report-2014. [Last updated on 2015 Dec].
- Horton R, Das P. Indian health: The path from crisis to progress. Lancet 2011;377:181-3.
- ;Garg CC, Karan AK. Reducing out-ofpocket expenditures to reduce poverty: A disaggregated analysis at rural-urban and state level in India. Health Policy Plan 2009;24:116-28.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03: National Cancer Institute, NIH Publication No. 09-5410, Revised June, 2010
- 13: Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: Variations in epidemiology and survival. Clin Breast Cancer 2005;6:391-401.
- Anderson BO, Jakesz R. Breast cancer issues in developing countries: An overview of the Breast Health Global Initiative. World J Surg 2008;32:2578-85.
- Porter P. “Westernizing” women's risks? Breast cancer in lower-income countries. N Engl J Med 2008;358:213-6.
- ;Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World J Surg 2007;31:1031-40.
- Saxena S, Szabo CI, Chopin S, Barjhoux L, Sinilnikova O, Lenoir G, et al. BRCA1 and BRCA2 in Indian breast cancer patients. Hum Mutat 2002;20:473-4.
- Murthy NS, Agarwal UK, Chaudhry K, Saxena S. A study on time trends in incidence of breast cancer – Indian scenario. Eur J Cancer Care (Engl) 2007;16:185-6.
- National Cancer Registry Programme: Consolidated Report of the Population Based Cancer Registries 1990-1996. Indian Council of Medical Research, New Delhi; 2001.
- Asthana S, Chauhan S, Labani S. Breast and cervical cancer risk in India: An update. Indian J Public Health 2014;58:5-10.
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. Available from: http://www.seer.cancer.gov/csr/1975_2012/. [Last Updated on 2015 Nov].
- Nikhra P, Patel S, Taviad D, Chaudhary S. Study of ER (Estrogen Receptor), PR (Progesterone Receptor) and HER-2/NEU (Human Epidermal Growth Factor Receptor) expression by immunohistochemistry in breast carcinoma. Int J Biomed Adv Res 2014;5:275-8.
- Zubeda S, Kaipa PR, Shaik NA, Mohiuddin MK, Vaidya S, Pavani B, et al. Her-2/neu status: A neglected marker of prognostication and management of breast cancer patients in India. Asian Pac J Cancer Prev 2013;14:2231-5.
- Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF. Hormone receptor status of breast cancer in India: A study of 798 tumours. Breast 2000;9:267-70.
- Raina V, Bhutani M, Bedi R, Sharma A, Deo SV, Shukla NK, et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J Cancer 2005;42:40-5.
- Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730-56.
- Gallagher CM, More K, Masaquel A, Kamath T, Guerin A, Ionescu-Ittu R, et al. Survival in patients with non-metastatic breast cancer treated with adjuvant trastuzumab in clinical practice. Springerplus 2016;5:395.
- Jana D, Mandal S, Mukhopadhyay M, Mitra D, Mukhopadhyay SK, Sarkar DK. Prognostic significance of HER-2/neu and survival of breast cancer patients attending a specialized breast clinic in Kolkata, Eastern India. Asian Pac J Cancer Prev 2012;13:3851-5.
- Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr., Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416-21.
- Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422-8.
- Available from: http://www.data.worldbank.org/indicator/NY.GDP.PCAP.CD. [Last updated on 2016 Dec].
- Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer 2011;48:391-6.
- Available from: http://www.drugscontrol.org/pdf/NLEM-2015.pdf. [Last updated on 2015 Dec].

| Figure 3:Flow chart of treatment schema of all human epidermal growth factor receptor 2-positive patients

| Figure 2:Five-year disease-free survival of all human epidermal growth factor receptor 2-positive patients

| Figure 3:Five-year disease-free survival in Stage II

| Figure 4:Five-year disease-free survival in Stage III

| Figure 5:Five-year overall survival of all human epidermal growth factor receptor 2-positive patients
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12.
- Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. ErbB-2 expression and its association with other biological parameters of breast cancer among Indian women. Indian J Cancer 2010;47:8-15.
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809-20.
- Malik K. United Nations Development Programme Report. Human Development Report; 2014. Available from: http://www.hdr.undp.org/en/content/humandevelopment-report-2014. [Last updated on 2015 Dec].
- Horton R, Das P. Indian health: The path from crisis to progress. Lancet 2011;377:181-3.
- ;Garg CC, Karan AK. Reducing out-ofpocket expenditures to reduce poverty: A disaggregated analysis at rural-urban and state level in India. Health Policy Plan 2009;24:116-28.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03: National Cancer Institute, NIH Publication No. 09-5410, Revised June, 2010
- 13: Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: Variations in epidemiology and survival. Clin Breast Cancer 2005;6:391-401.
- Anderson BO, Jakesz R. Breast cancer issues in developing countries: An overview of the Breast Health Global Initiative. World J Surg 2008;32:2578-85.
- Porter P. “Westernizing” women's risks? Breast cancer in lower-income countries. N Engl J Med 2008;358:213-6.
- ;Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World J Surg 2007;31:1031-40.
- Saxena S, Szabo CI, Chopin S, Barjhoux L, Sinilnikova O, Lenoir G, et al. BRCA1 and BRCA2 in Indian breast cancer patients. Hum Mutat 2002;20:473-4.
- Murthy NS, Agarwal UK, Chaudhry K, Saxena S. A study on time trends in incidence of breast cancer – Indian scenario. Eur J Cancer Care (Engl) 2007;16:185-6.
- National Cancer Registry Programme: Consolidated Report of the Population Based Cancer Registries 1990-1996. Indian Council of Medical Research, New Delhi; 2001.
- Asthana S, Chauhan S, Labani S. Breast and cervical cancer risk in India: An update. Indian J Public Health 2014;58:5-10.
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. Available from: http://www.seer.cancer.gov/csr/1975_2012/. [Last Updated on 2015 Nov].
- Nikhra P, Patel S, Taviad D, Chaudhary S. Study of ER (Estrogen Receptor), PR (Progesterone Receptor) and HER-2/NEU (Human Epidermal Growth Factor Receptor) expression by immunohistochemistry in breast carcinoma. Int J Biomed Adv Res 2014;5:275-8.
- Zubeda S, Kaipa PR, Shaik NA, Mohiuddin MK, Vaidya S, Pavani B, et al. Her-2/neu status: A neglected marker of prognostication and management of breast cancer patients in India. Asian Pac J Cancer Prev 2013;14:2231-5.
- Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF. Hormone receptor status of breast cancer in India: A study of 798 tumours. Breast 2000;9:267-70.
- Raina V, Bhutani M, Bedi R, Sharma A, Deo SV, Shukla NK, et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J Cancer 2005;42:40-5.
- Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730-56.
- Gallagher CM, More K, Masaquel A, Kamath T, Guerin A, Ionescu-Ittu R, et al. Survival in patients with non-metastatic breast cancer treated with adjuvant trastuzumab in clinical practice. Springerplus 2016;5:395.
- Jana D, Mandal S, Mukhopadhyay M, Mitra D, Mukhopadhyay SK, Sarkar DK. Prognostic significance of HER-2/neu and survival of breast cancer patients attending a specialized breast clinic in Kolkata, Eastern India. Asian Pac J Cancer Prev 2012;13:3851-5.
- Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr., Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416-21.
- Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422-8.
- Available from: http://www.data.worldbank.org/indicator/NY.GDP.PCAP.CD. [Last updated on 2016 Dec].
- Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer 2011;48:391-6.
- Available from: http://www.drugscontrol.org/pdf/NLEM-2015.pdf. [Last updated on 2015 Dec].


 PDF
PDF  Views
Views  Share
Share

