Treatment Outcomes in Advanced Biliary Tract Cancers: Single Institution Retrospective Analysis
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(04): 370-376
DOI: DOI: 10.1055/s-0044-1787750
Abstract
Purpose Biliary tract cancers (BTCs), particularly gallbladder cancers (GBCs), are prevalent in India. Yet there are limited data on treatment outcomes. To bridge this gap, we performed an analysis of advanced BTC treatment outcomes at our institute, seeking to offer insights into real-world scenario.
Materials and Methods This is a retrospective study comprising advanced BTC patients treated at our institute from January 2015 to March 2023. We assessed demographics, treatment approaches, progression-free survival (PFS), overall survival (OS), and associated toxicities.
Results Of the 411 patients analyzed, the majority were GBC (67.3%, n = 277), while the rest were cholangiocarcinoma (CCA) (32.6%, n = 134). The median age of study population was 56 years. Palliative chemotherapy was administered in 85% (n = 349) of all patients. Gemcitabine–cisplatin doublet was the most commonly used chemotherapy regimen (80.2%, n = 280). Platinum doublets yielded higher response rates compared with single-agent/nonplatinum chemotherapy (60 vs. 30%, n = 133). The median PFS was 4 months. The median OS was 8 months with platinum doublets and 5 months with single-agent/nonplatinum chemotherapy (hazard ratio [HR]: 0.60, 95% confidence interval: [CI] 0.43–0.84, p = 0.0001). OS was no different based on the type of platinum agent used. Patients receiving multiple lines of treatment lived longer compared with those who received single line only (14 vs. 6 months, respectively, HR: 0.36, 95% CI: 0.28–0.45, p < 0.0001). Significant prognostic factors for OS were treatment with chemotherapy, platinum doublets, platinum exposure in first line, and treatment beyond first line. Grade 3 or 4 adverse effects seen were anemia (13.9%, n = 36), vomiting (4.2%, n = 11), diarrhea (3.4%, n = 9), thrombocytopenia (3.4%, n = 9), and febrile neutropenia (3.1%, n = 8).
Conclusion This analysis confirms that chemotherapy is beneficial for advanced BTC. Platinum-based doublets are more effective than single agents. There is no significant difference between cisplatin and oxaliplatin. Patients who received multiple lines of treatment had better OS.
Keywords
Authors' Contributions
N.P. contributed to conceptualization, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. S.J.R. contributed to conceptualization, design, definition of intellectual content, clinical studies, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. S., M.V.T.K.M., P.K., R.P., P.L., S.K., R.T, K.S., and S.R.K. contributed to data acquisition, manuscript preparation, and manuscript review. K.B. contributed to data acquisition and manuscript review. D.G. contributed to data analysis, statistical analysis, and manuscript review.
Patient Consent
Patient consent is not required due to the retrospective nature of the study.
Publication History
25 June 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Presentation and Outcomes with First-Line Chemotherapy in Advanced Cholangiocarcinomas—A Relatively Rare Component of Biliary Tract Cancers in IndiaPrabhat G. Bhargava, South Asian Journal of Cancer, 2020
- Multidisciplinary approach for biliary tract cancer after progression to first-line therapy with gemcitabine and cisplatin: post-progression survival and safety...Christian Müller, Zeitschrift für Gastroenterologie, 2023
- Treatment Outcomes of Advanced Cholangiocarcinoma: A Single-Center Experience from IndiaNiranjan Vijayaraghavan, et al., South Asian Journal of Cancer, 2022
- Pattern of Care and Outcomes of Gallbladder Cancer Patients: Retrospective Study from a High Incidence Region in IndiaLakhan Kashyap, South Asian Journal of Cancer, 2023
- GI-Interventional Oncology—CholangiocarcinomaSarah B. White, et al., Digestive Disease Interventions, 2024
- Screening strategy on precision prevention strategies for three types of malignant tumors<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
</svg> CAI Shiliang, Featured Articles on COVID from Shanghai Journal of Preventive Medicine - Comparison of incidence and survival of liver cancer between permanent residents in Yangpu District of Shanghai and Qidong City of Jiangsu Province during 2002-...HAN Xue 1, Featured Articles on COVID from Shanghai Journal of Preventive Medicine
- Factors affecting the prognosis of rectal cancer in elderly patientsWANG Chun-liang, Featured Articles on COVID from Shanghai Journal of Preventive Medicine
- Survival analysis and compliance nomogram model construction of patients with non-small cell lung cancer over 65 years old after chemotherapyZHANG Man, Featured Articles on COVID from Shanghai Journal of Preventive Medicine, 2024
- Morbidity and Mortality analysis on malignant tumor from 2006 to 2011 in Xiacheng District of Hangzhou CityZHAO Qi, Featured Articles on COVID from Shanghai Journal of Preventive Medicine
Abstract
Purpose Biliary tract cancers (BTCs), particularly gallbladder cancers (GBCs), are prevalent in India. Yet there are limited data on treatment outcomes. To bridge this gap, we performed an analysis of advanced BTC treatment outcomes at our institute, seeking to offer insights into real-world scenario.
Materials and Methods This is a retrospective study comprising advanced BTC patients treated at our institute from January 2015 to March 2023. We assessed demographics, treatment approaches, progression-free survival (PFS), overall survival (OS), and associated toxicities.
Results Of the 411 patients analyzed, the majority were GBC (67.3%, n = 277), while the rest were cholangiocarcinoma (CCA) (32.6%, n = 134). The median age of study population was 56 years. Palliative chemotherapy was administered in 85% (n = 349) of all patients. Gemcitabine–cisplatin doublet was the most commonly used chemotherapy regimen (80.2%, n = 280). Platinum doublets yielded higher response rates compared with single-agent/nonplatinum chemotherapy (60 vs. 30%, n = 133). The median PFS was 4 months. The median OS was 8 months with platinum doublets and 5 months with single-agent/nonplatinum chemotherapy (hazard ratio [HR]: 0.60, 95% confidence interval: [CI] 0.43–0.84, p = 0.0001). OS was no different based on the type of platinum agent used. Patients receiving multiple lines of treatment lived longer compared with those who received single line only (14 vs. 6 months, respectively, HR: 0.36, 95% CI: 0.28–0.45, p < 0.0001). Significant prognostic factors for OS were treatment with chemotherapy, platinum doublets, platinum exposure in first line, and treatment beyond first line. Grade 3 or 4 adverse effects seen were anemia (13.9%, n = 36), vomiting (4.2%, n = 11), diarrhea (3.4%, n = 9), thrombocytopenia (3.4%, n = 9), and febrile neutropenia (3.1%, n = 8).
Conclusion This analysis confirms that chemotherapy is beneficial for advanced BTC. Platinum-based doublets are more effective than single agents. There is no significant difference between cisplatin and oxaliplatin. Patients who received multiple lines of treatment had better OS.
Keywords
advanced biliary tract cancers - first-line chemotherapy - outcomes
Introduction
Biliary tract cancers (BTCs) are rare and vary in prevalence worldwide.[1] India contributes up to 10%. of global BTC burden. Among BTC, gallbladder cancers (GBCs) are more prevalent in India (north vs. south India: 21.5 vs. 0.7/100,000 population).[2] Recent data from the Indian Council of Medical Research show GBC among the top 10 cancers in men in Assam and Jammu and Kashmir (2.6–6% of all cases) and women across northern and northeast regions. Cholangiocarcinomas (CCAs) are rare bile duct tumors, affecting less than 6 in 100,000 people. Their occurrence varies by geographic location, possibly due to different risk factors.[3] Clinical presentation of BTC is quite nonspecific during early stages in the majority. This leads to a delay in diagnosis, presentation in advanced stages making them ineligible for any curative treatments and thus overall poor outcomes.
Gemcitabine with cisplatin or oxaliplatin remains the initial chemotherapy regimen of choice in advanced BTC.[4] [5] In a recent trial, there was no benefit to the addition of a third drug, paclitaxel to the standard doublet regimen.[6] Across Indian studies, the doublet combination has shown a median overall survival (OS) of 8.5 months.[7] Although two new randomized clinical trials have shown a modest survival benefit with the addition of an immune checkpoint inhibitor to the gemcitabine–platinum doublet, its applicability to a majority of our patients is questionable because of affordability issues.[8] [9] Herein, we did a retrospective analysis of patients with advanced BTC treated at our institute to reflect real-world outcomes.
Materials and Methods
Database and Patient Population
This is a comprehensive analysis of treatment-naive patients with locally advanced/unresectable or metastatic adenocarcinoma of the biliary tract who presented to our institute from January 2015 to March 2023. Patient data were extracted from medical records and the hospital's electronic database. Patients deemed eligible were administered platinum-based doublet or single-agent chemotherapy at the discretion of the treating physician. Unfit patients were offered best supportive care (BSC) alone.
Outcome Variables
The parameters analyzed included demographics, treatment patterns, and outcomes. Data collected included specific regimens used (single agent vs. doublets, cisplatin/carboplatin vs. oxaliplatin), the number of lines of chemotherapy, objective response rates (ORR), progression-free survival (PFS), OS, and toxicity profile. Response evaluation to treatment was done after three or four cycles of chemotherapy or earlier at the discretion of treating physician. A clinical assessment followed by computed tomography (CT) scan, utilizing the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) was performed. Response rates and clinical benefit rates (CBRs) were reported as percentages. The primary outcome variables analyzed were ORR, PFS, and OS.
PFS was calculated from the date of starting therapy to progressive disease, follow-up loss, chemotherapy discontinuation due to adverse events of grade 3 or 4 severity, or death secondary to any cause. OS was determined from the date of diagnosis until the death of the patient or the last follow-up, as confirmed through hospital records or telephonic contact, whichever was feasible. Treatment toxicity was assessed as per National Cancer Institute—Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Analysis
Descriptive statistics (median values, frequencies, and percentages) were employed to characterize categorical variables such as age, gender distribution, treatment modalities, treatment response, and toxicities. Median PFS and OS were estimated using the Kaplan–Meier method. Hazard ratios (HR) for survival and 95% confidence intervals (CIs) were computed using the Mantel–Cox's test. Prognostic factors for PFS and OS were assessed using the log-rank test. Univariate analysis was performed using chi-square test and Fisher's exact test. Multivariate analysis by multiple logistic regression method was performed using SPSS 21.0 software (IBM, Armonk, New York, United States). A p-value less than 0.05 is considered significant.
Ethical Approval
This study received ethical approval from the Basavatarakam Indo-American Cancer Hospital & Research Institute, Institutional Ethics Committee Board (BIACH&RI IEC, Reg. no.: ECR/7/Inst/AP/2013/RR-20, protocol no.: IEC/2023/12, date: January 1, 2023). All procedures performed were by the ethical standards of the institution and/or national research committee. They were also in concordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards apart from Good Clinical Practice guidelines for the International Conference on Harmonization.
Results
Part I: Demographics and Patient Characteristics
A total of 411 patients with advanced BTC were analyzed. There were 277 cases (67.3%) of GBC and 134 cases (32.6%) of CCA. The median age of the study population was 56 years (range: 18–76 years). There was a slight preponderance of females (54.8%, n = 152) in the GBC and males (61.1%, n = 82) in the CCA subgroup. Liver (83.4%, n = 343) and nonregional nodes (83.2%, n = 342) were the most common sites of metastases, followed by the skeleton (4.8%, n = 20) and lungs (4.2%, n = 18). Among CCA, intrahepatic CCA was the most frequent subsite seen (88%, n = 118).
Part II: Treatment Data
|
Whole cohort |
Gallbladder carcinoma |
Cholangiocarcinoma |
|
|---|---|---|---|
|
Total patients (n) |
411 |
277 (67.3%) |
134 (32.6%) |
|
Median age (y) |
56 |
56 |
56 |
|
Range (y) |
18–76 |
23–80 |
18–76 |
|
Male |
207 (50.3%) |
125 (45.1%) |
82 (61.1%) |
|
Female |
204 (49.7%) |
152 (54.8%) |
52 (38.8%) |
|
ERCP + stent |
46 (11.19%) |
30 (10.8%) |
16 (11.9%) |
|
Histologic differentiation |
|||
|
Well |
38 (9.2%) |
23 (8.3%) |
15 (11.19%) |
|
Moderate |
265 (64.4%) |
174 (62.8%) |
91 (67.9%) |
|
Poor |
108 (26.2%) |
80 (28.8%) |
28 (20.8%) |
|
Lines of treatment received |
|||
|
0 (BSC) |
62 (15.08%) |
34 (12.2%) |
28 (20.8%) |
|
1 |
264 (64.23%) |
184 (66.4%) |
80 (59.7%) |
|
2 |
61 (14.84%) |
45 (16.2%) |
16 (11.9%) |
|
≥3 |
24 (5.83%) |
14 (5.05%) |
10 (7.4%) |
|
Range |
0–6 |
0–6 |
0–6 |
|
First-line chemotherapy received |
349/411 (84.9%) |
243/277 (87.7%) |
106/134 (79.1%) |
|
Platinum-based combination |
280/349 (80.2%) |
206/243 (84.7%) |
74/106 (69.8%) |
|
Gemcitabine–platinum |
269 (96.04%) |
197 (95.6%) |
72 (97.2%) |
|
5FU platinum |
11 (3.9%) |
9 (4.3%) |
2 (2.7%) |
|
Single-agent chemotherapy |
69/349 (19.7%) |
37/243 (15.2%) |
32/106 (30.1%) |
|
5FU |
23 (33.3%) |
12 (32.4%) |
11 (34.3%) |
|
Gemcitabine |
46 (66.6%) |
25 (67.5%) |
21 (65.6%) |
About 87.7%. (n = 243) in the GBC and 79.1%. (n = 106) in the CCA group received palliative chemotherapy. Platinum doublets were used in 84.7%. (n = 206) of GBC and 69.8%. (n = 74) of CCA patients. The preferred platinum partner was gemcitabine (GBC: 95.6%, n = 197 vs. CCA: 97%, n = 72). Single-agent chemotherapy was used less frequently (GBC: 15.2%, n = 37 vs. CCA: 30%, n = 32). Gemcitabine was the preferred single agent when used (GBC: 67.5%. n = 25 vs. CCA: 65.6%, n = 21). Only 32.2%. of all patients (entire cohort: n = 85, GBC: n = 59, CCA: n = 26) received treatment beyond first line. As second-line treatments, platinum doublets (n = 35, 41.1%), FOLFIRI (n = 21, 24.7%.), irinotecan (n = 13, 15.2%.), capecitabine (n = 8, 9.4%.), and others were used.
Part III: Overall Survival
The median OS for the entire study group, GBC group, and CCA group was 6 months ([Fig. 1]).
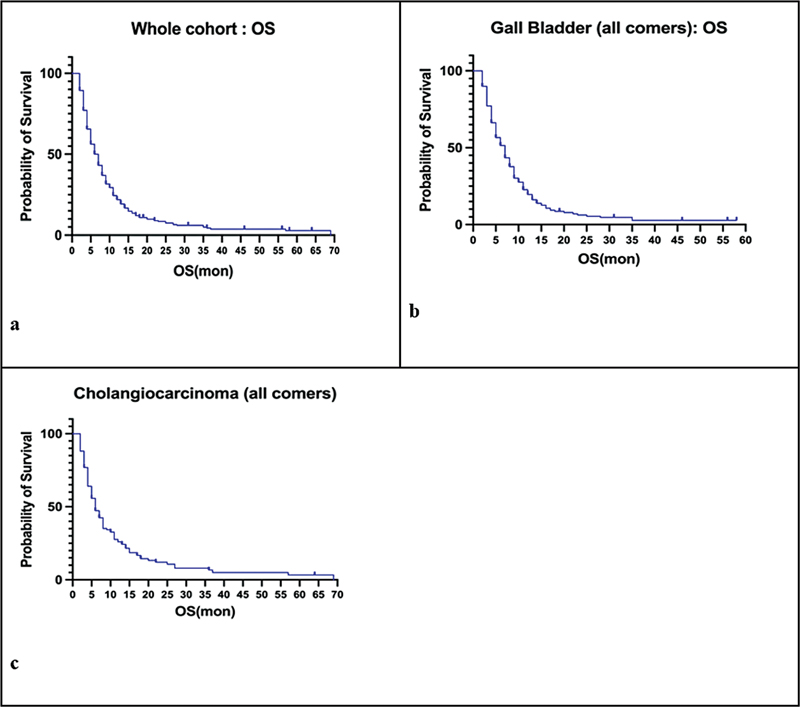
Fig 1: Kaplan–Meier survival curves depicting overall survival (OS) for the whole cohort (a), gallbladder carcinoma (b), and cholangiocarcinoma (c).
Part IV: Progression-Free Survival
The median PFS was 4 months (range: 3–16 months), similar across the entire cohort and individual subgroups ([Fig. 2]). Platinum doublets, in comparison to single-agent/nonplatinum chemotherapy, resulted in a better PFS in the entire cohort (5 vs. 3 months, HR: 0.61, 95%. CI: 0.41–0.91, p < 0.0001) as well as in the CCA group (5 vs. 2 months, HR: 0.53, 95%. CI: 0.29–0.98, p = 0.004). However, the same was not seen in the GBC group (5 vs. 3 months, HR: 0.69, 95%. CI: 0.41–1.17, p = NS). There was no difference in PFS between cisplatin and oxaliplatin.
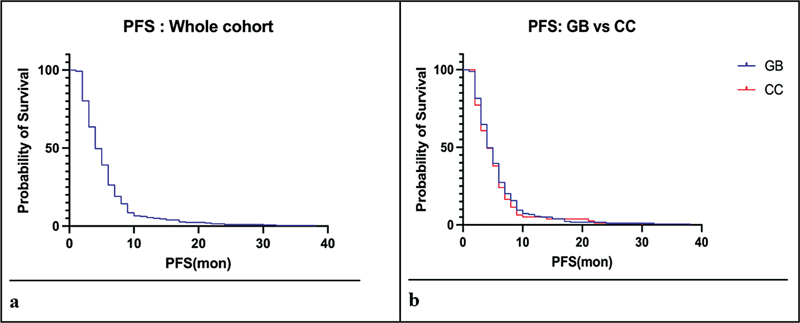
Fig 2 : Kaplan–Meier survival curves depicting progression-free survival (PFS) for the whole cohort (a), and gallbladder (GB) and cholangiocarcinoma (CC) (b).
Part V: Response Evaluation and Clinical Benefit Assessment
Response evaluation as per RECIST criteria was feasible in 258 patients (73.9%.). Progression of disease (PD) was seen in 26.3%. (n = 68), while 56.5% (n = 146) had a partial response (PR). Stable disease was observed in 16.6%. (n = 43) of patients. Only one patient had a complete response. The CBR was 73.4%. (n = 191).
Platinum doublets demonstrated higher responses in comparison to single/nonplatinum agent (PR: 61.2%, n = 133 vs. 31.7%, n = 13). Disease progression was also lower with doublets compared with single-agent/nonplatinum chemotherapy (PD: 22.1%, n = 48 vs. 48.7%, n = 20). The response rates did not differ significantly based on the type of platinum agent used.
Part VI: Toxicity Data (Only CTCAE Grade 3 or 4)
About 4.2%. (n = 11) patients experienced chemotherapy-induced nausea/vomiting, while 3.4%.(n = 9) had diarrhea. Anemia was seen in 13.9%. (n = 36), more so when gemcitabine–platinum combination was used. Febrile neutropenia was noted in 3.1%. (n = 8) and thrombocytopenia in 3.4%.. (n = 9) of cases. Biliary sepsis was documented in 3.8%. (n = 10) patients. Transaminitis secondary to oxaliplatin was present in 4.2%. (n = 11) cases. Change to oxaliplatin/carboplatin secondary to cisplatin-induced renal dysfunction was seen in four patients. Neuropathy was observed in 3.8%. (n = 10) of cases.
Part VII: Prognostic Factors Affecting Overall Survival
|
Dependent variable |
Mean square |
Partial eta squared |
p-Value |
|
|---|---|---|---|---|
|
Age |
<50>50 |
0.213 |
0.085 |
0.376 |
|
Sex |
Male vs. female |
0.222 |
0.071 |
0.665 |
|
Disease type |
GBC vs. CCA |
0.296 |
0.108 |
0.082 |
|
Biliary obstruction |
Yes. vs. no |
0.111 |
0.090 |
0.296 |
|
Treatment received |
Chemotherapy vs. BSC alone |
0.750 |
0.476 |
<0> |
|
Platinum doublet vs. single/nonplatinum agent |
2.875 |
0.423 |
<0> |
|
|
Cisplatin/carboplatin vs. oxaliplatin |
2.710 |
0.284 |
0.284 |
|
|
Single line vs. multiple lines |
2.390 |
0.351 |
<0> |
|
Discussion
Drawbacks of the Study
The drawbacks of the study were as follows:
Retrospective study.
Heterogeneity of treatments.
Missing data regarding grade 1 or 2 toxicity.
Missing quality of life analysis.
Conclusion
This retrospective analysis confirms the benefit of chemotherapy in advanced BTC. Platinum-based doublets are more effective than single agents. There is no difference between cisplatin and oxaliplatin. Patients who received more than one line of treatment had better OS.
Conflict of Interest
None declared.
Authors' Contributions
N.P. contributed to conceptualization, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. S.J.R. contributed to conceptualization, design, definition of intellectual content, clinical studies, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. S., M.V.T.K.M., P.K., R.P., P.L., S.K., R.T, K.S., and S.R.K. contributed to data acquisition, manuscript preparation, and manuscript review. K.B. contributed to data acquisition and manuscript review. D.G. contributed to data analysis, statistical analysis, and manuscript review.
Patient Consent
Patient consent is not required due to the retrospective nature of the study.
References
- Randi G, Malvezzi M, Levi F. et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009; 20 (01) 146-159
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006; 118 (07) 1591-1602
- Banales JM, Cardinale V, Carpino G. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016; 13 (05) 261-280
- Ramaswamy A, Ostwal V, Pinninti R. et al. Gemcitabine-cisplatin versus gemcitabine-oxaliplatin doublet chemotherapy in advanced gallbladder cancers: a match pair analysis. J Hepatobiliary Pancreat Sci 2017; 24 (05) 262-267
- Patkar S, Ostwal V, Ramaswamy A. et al. Emerging role of multimodality treatment in gall bladder cancer: outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol 2018; 117 (03) 372-379
- Shroff RT, Guthrie KA, Scott AJ. et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol 2023; 41 (04) A490
- Sharma A, Kalyan Mohanti B, Pal Chaudhary S. et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: results of a phase III randomised controlled trial. Eur J Cancer 2019; 123: 162-170
- Oh DY, He AR, Qin S. et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol 2022; 40 (4, suppl): 378
- Kelley RK, Ueno M, Yoo C. et al; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 401 (10391): 1853-1865
- Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Chin Clin Oncol 2019; 8 (04) 33
- Dutta A, Mungle T, Chowdhury N. et al. Characteristics and outcomes of gallbladder cancer patients at the Tata Medical Center, Kolkata 2017-2019. Cancer Med 2023; 12 (08) 9293-9302
- Mehrotra R, Tulsyan S, Hussain S. et al. Genetic landscape of gallbladder cancer: global overview. Mutat Res Rev Mutat Res 2018; 778: 61-71
- Patkar S, Gupta V, Khobragade K, Goel M. The reality of cholangiocarcinoma in India- real world data from a tertiary referral centre. HPB (Oxford) 2022; 24 (09) 1511-1518
- Nair P, Rao H, Koshy AK. et al. Cholangiocarcinoma in South India: unprecedented, unanticipated and underreported. Int J Community Med Public Health 2021; 8 (08) 3854-3863
- Valle JW, Furuse J, Jitlal M. et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014; 25 (02) 391-398
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014; 60 (01) 13-23
- Amit RK, Anadure HP, Singh R. et al. A study on the clinical profile and treatment outcomes in gallbladder carcinoma from northern India. Oncology Journal of India 2020; 4 (03) 128-132
- Sharma A, Dwary AD, Mohanti BK. et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010; 28 (30) 4581-4586
- André T, Tournigand C, Rosmorduc O. et al; GERCOR Group. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004; 15 (09) 1339-1343
- Javle M, Borad MJ, Azad NS. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2021; 22 (09) 1290-1300
- Lee CK, Chon HJ, Cheon J. et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol 2023; 8 (01) 56-65
- Ohba A, Morizane C, Kawamoto Y. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). Published online 2022; 4006-4006
- Bhargava PG, Kumar A, Simha V. et al. Presentation and outcomes with first-line chemotherapy in advanced cholangiocarcinomas-A relatively rare component of Biliary tract cancers in India. South Asian J Cancer 2020; 9 (04) 209-212
- Anadure R, Sreen A, Singh HP. et al. A study on the clinical profile and treatment outcomes in gallbladder carcinoma from Northern India. Oncol J India 2020; 4 (03) 128-132
Address for correspondence
Publication History
25 June 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Presentation and Outcomes with First-Line Chemotherapy in Advanced Cholangiocarcinomas—A Relatively Rare Component of Biliary Tract Cancers in IndiaPrabhat G. Bhargava, South Asian Journal of Cancer, 2020
- Multidisciplinary approach for biliary tract cancer after progression to first-line therapy with gemcitabine and cisplatin: post-progression survival and safety...Christian Müller, Zeitschrift für Gastroenterologie, 2023
- Treatment Outcomes of Advanced Cholangiocarcinoma: A Single-Center Experience from IndiaNiranjan Vijayaraghavan, et al., South Asian Journal of Cancer, 2022
- Pattern of Care and Outcomes of Gallbladder Cancer Patients: Retrospective Study from a High Incidence Region in IndiaLakhan Kashyap, South Asian Journal of Cancer, 2023
- GI-Interventional Oncology—CholangiocarcinomaSarah B. White, et al., Digestive Disease Interventions, 2024
- Epidemiology of biliary tract cancer in China: A narrative review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, sin...Guo-Ming Shi, Signal Transduction and Targeted Therapy, 2023
- Clinical and biomarker analyses of SHR-1701 combined with famitinib in patients with previously treated advanced biliary tract cancer or pancreatic ductal adeno...Lixia Yi, et al., Signal Transduction and Targeted Therapy, 2024
- Cholangiocarcinoma combined with biliary obstruction: an exosomal circRNA signature for diagnosis and early recurrence monitoring<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
</svg> Ningyuan Wen, et al., Signal Transduction and Targeted Therapy, 2024 - Biliary diseases: diagnostic and therapeutic challenges.

Fig 1: Kaplan–Meier survival curves depicting overall survival (OS) for the whole cohort (a), gallbladder carcinoma (b), and cholangiocarcinoma (c).

Fig 2 : Kaplan–Meier survival curves depicting progression-free survival (PFS) for the whole cohort (a), and gallbladder (GB) and cholangiocarcinoma (CC) (b).
References
- Randi G, Malvezzi M, Levi F. et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009; 20 (01) 146-159
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006; 118 (07) 1591-1602
- Banales JM, Cardinale V, Carpino G. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016; 13 (05) 261-280
- Ramaswamy A, Ostwal V, Pinninti R. et al. Gemcitabine-cisplatin versus gemcitabine-oxaliplatin doublet chemotherapy in advanced gallbladder cancers: a match pair analysis. J Hepatobiliary Pancreat Sci 2017; 24 (05) 262-267
- Patkar S, Ostwal V, Ramaswamy A. et al. Emerging role of multimodality treatment in gall bladder cancer: outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol 2018; 117 (03) 372-379
- Shroff RT, Guthrie KA, Scott AJ. et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol 2023; 41 (04) A490
- Sharma A, Kalyan Mohanti B, Pal Chaudhary S. et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: results of a phase III randomised controlled trial. Eur J Cancer 2019; 123: 162-170
- Oh DY, He AR, Qin S. et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol 2022; 40 (4, suppl): 378
- Kelley RK, Ueno M, Yoo C. et al; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 401 (10391): 1853-1865
- Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Chin Clin Oncol 2019; 8 (04) 33
- Dutta A, Mungle T, Chowdhury N. et al. Characteristics and outcomes of gallbladder cancer patients at the Tata Medical Center, Kolkata 2017-2019. Cancer Med 2023; 12 (08) 9293-9302
- Mehrotra R, Tulsyan S, Hussain S. et al. Genetic landscape of gallbladder cancer: global overview. Mutat Res Rev Mutat Res 2018; 778: 61-71
- Patkar S, Gupta V, Khobragade K, Goel M. The reality of cholangiocarcinoma in India- real world data from a tertiary referral centre. HPB (Oxford) 2022; 24 (09) 1511-1518
- Nair P, Rao H, Koshy AK. et al. Cholangiocarcinoma in South India: unprecedented, unanticipated and underreported. Int J Community Med Public Health 2021; 8 (08) 3854-3863
- Valle JW, Furuse J, Jitlal M. et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014; 25 (02) 391-398
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014; 60 (01) 13-23
- Amit RK, Anadure HP, Singh R. et al. A study on the clinical profile and treatment outcomes in gallbladder carcinoma from northern India. Oncology Journal of India 2020; 4 (03) 128-132
- Sharma A, Dwary AD, Mohanti BK. et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010; 28 (30) 4581-4586
- André T, Tournigand C, Rosmorduc O. et al; GERCOR Group. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004; 15 (09) 1339-1343
- Javle M, Borad MJ, Azad NS. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2021; 22 (09) 1290-1300
- Lee CK, Chon HJ, Cheon J. et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol 2023; 8 (01) 56-65
- Ohba A, Morizane C, Kawamoto Y. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). Published online 2022; 4006-4006
- Bhargava PG, Kumar A, Simha V. et al. Presentation and outcomes with first-line chemotherapy in advanced cholangiocarcinomas-A relatively rare component of Biliary tract cancers in India. South Asian J Cancer 2020; 9 (04) 209-212
- Anadure R, Sreen A, Singh HP. et al. A study on the clinical profile and treatment outcomes in gallbladder carcinoma from Northern India. Oncol J India 2020; 4 (03) 128-132


 PDF
PDF  Views
Views  Share
Share

