Tumor bed boost in breast cancer: Brachytherapy versus electron beam
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 257-263
DOI: DOI: 10.4103/0971-5851.125238
Abstract
Background: The prospective study aimed to evaluate the effectiveness of Electron beam or HDR 192Ir Interstitial Implant used as a boost in breast Conservation cases after completion of EBRT. The two therapeutic modalities were compared in terms of the following parameters; i.e. cosmesis, optimization of tumor bed boost, local control, toxicity, and DFS. Materials and Methods: The EBRT dose used was 50 Gy in 25 fractions over 5 weeks time. Target delineation of boost treatment was done by CT scan or by high resolution USG. EBRT will be immediately followed by local boost at the primary tumor bearing site of breast with 8 to12 MeV electron beam to a dose of 15 Gy in 6 fractions (Arm A) or with HDR 192Ir interstitial brachytherapy to a dose of 15 Gy in 3 fractions at 6 hours apart (Arm B). Results: The excellent cosmesis achieved with electron beam therapy in Arm A was found to be statistically significant (P = 0.025). Local relapse was absent in both the arms. One distant metastasis occurred in Arm A within 10 months of initiation of treatment and one distant metastasis in Arm B came out within 3 months of starting of therapy. Conclusion: The study has shown good cosmetic result with electron boost and 100% local control with both the technique. However if there is a more number of patients with longer period of follow up we could have got the actual picture to verify our results and assess long term survival data.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The prospective study aimed to evaluate the effectiveness of Electron beam or HDR 192Ir Interstitial Implant used as a boost in breast Conservation cases after completion of EBRT. The two therapeutic modalities were compared in terms of the following parameters; i.e. cosmesis, optimization of tumor bed boost, local control, toxicity, and DFS.
Materials and Methods:
The EBRT dose used was 50 Gy in 25 fractions over 5 weeks time. Target delineation of boost treatment was done by CT scan or by high resolution USG. EBRT will be immediately followed by local boost at the primary tumor bearing site of breast with 8 to12 MeV electron beam to a dose of 15 Gy in 6 fractions (Arm A) or with HDR 192Ir interstitial brachytherapy to a dose of 15 Gy in 3 fractions at 6 hours apart (Arm B).
Results:
The excellent cosmesis achieved with electron beam therapy in Arm A was found to be statistically significant (P = 0.025). Local relapse was absent in both the arms. One distant metastasis occurred in Arm A within 10 months of initiation of treatment and one distant metastasis in Arm B came out within 3 months of starting of therapy.
Conclusion:
The study has shown good cosmetic result with electron boost and 100% local control with both the technique. However if there is a more number of patients with longer period of follow up we could have got the actual picture to verify our results and assess long term survival data.
INTRODUCTION
The treatment of breast cancer has seen significant changes over the past century. Since the beginning of the 20th century based on Halsted's hypothesis of tumor spread, it was incorrectly thought that wider the surgical extirpation, the greater the chance of cure.[1] McWhirter[2] popularized a lesser surgical procedure (total mastectomy) in combination with irradiation to the chest wall and regional lymphatic, a technique that yielded results comparable with those of radical mastectomy.[3,4] Keynes[5] in 1929 and 1937, combined conservation surgery (ranging from biopsy to wide local tumor excisions to segmental mastectomy or definitive irradiation.[6,7,8,9] This approach progressively gained acceptance in the United States since the early 1980[10] and then globally. Breast conservation treatment have survival rates at least as high as patients allocated to mastectomy in early stage breast cancers[11] not all women are candidates for this approach and some require mastectomy as part of their treatment. Present evidence suggests that the sequence is of no consequence for survival.[12,13,14] The response of locally advanced breast cancer to neo adjuvant chemotherapy offers these patients the chance of breast conservation.[15] The goal of sparing the breast in breast conservation treatment is substantially less likely to be accomplished without the addition of breast irradiation. NSABP B-06 trial demonstrated recurrence in the breast in over one third of women treated without irradiation over the next 10 years after breast conservation surgery. Data from Early Breast Cancer Trialists’ Collaborative Group meta-analysis also showed that the group not receiving radiation had increased mortality from breast cancer and increased overall mortality.[16] Today post-operative radiotherapy is mandatory following breast conservation surgery irrespective of tumor size, the number of positive axillary lymph nodes, or the histological grade.[17,18] Irradiation after lumpectomy is effective in reducing the risk of ipsilateral breast cancer recurrence. The 20 years update of NSABP B-06 reported 39.2% local failure without irradiation compared with 14.3% with irradiation.[19] Such recurrence is typically in the immediate vicinity of the lumpectomy site, termed true recurrence or marginal miss.[20] In the European Organization for Research and Treatment of Cancer (EORTC) “boost no boost” trial designated to clarify the role of the boost also showed the magnitude of the benefit was greatest in patients 40 years of age or younger, in whom local recurrence was halved by the addition of a boost dose.[21] EBC constitutes only about 30% of the breast cancer load in our country with the majority diagnosed in clinical Stage III.[22] The use of high dose rate (HDR) interstitial brachytherapy or electron for the boost purpose is relatively new having a history of around a decade only. Many institutions prefer electron beam boost because of its relative ease in set up, out-patient setting, lower cost, decreased time demands on the physician and excellent results compared with Iridium implants. Brachytherapy boost technique has the advantage of decreased skin dose, potential radiobiological advantages compared with electron beam boost therapy. Brachytherapy is preferred in women with large breasts and deep tumors because the integral dose with electrons is high and there can be exit dose into the lung. Optimization of the cosmetic outcome can be done by choosing the machinery, energy of the beam, dose per fraction, total dose and optimal treatment plan with the use of wedged compensators individually chosen for the patient.
Delivery of conventional fractionation of 180-200c Gy per fraction when compared with doses above 250c Gy per fraction minimizes reaction of the skin and breast tissue and optimizes the possibility of a good cosmetic result while effectively irradiating residual neoplastic cells.[23,24,25,26] If the dose to the whole breast surpasses 50-60 Gy, an increased rate of long-term breast edema and compromised cosmesis are risked. At doses above 70 Gy, fibrosis, pain and retraction may be seen. Electron energy should be such that it reaches the tumor bed while minimizing dose to the overlying (skin) and underlying (chest-wall and lung) anatomy.[27] Using an iridium (brachytherapy) boost has worsened cosmesis in a few reports.[28,29]
MATERIALS AND METHODS
The prospective study was performed from 9th October 2005 to 31st December 2007. Breast cancer patients after registration in our hospital, were first clinically examined thoroughly for staging, bilateral film mammogram was done next for documentation of exact extent of primary tumor. Pathological confirmation was followed by complete metastatic work-up. The patient who could fulfill the breast conservation criteria underwent conservation surgery and full course of chemotherapy. After the breast conserving surgery (BCS), the patients were randomized for radiotherapy assigned to Arm A or Arm B. Each patient was allotted with a computerized randomization number in an unrestricted randomization process. The study was approved by the Institutional Ethics Committee beforehand and all the human model study guidelines were maintained according to international criteria.
Arm-A patients received external beam radiotherapy (EBRT) from 6 MV linear accelerator. In all the patients, the regional nodes were irradiated with a direct anterior field while tangential beams were used to treat the whole breast. The radiation dose used was 50 Gy in 25 fractions over 5 weeks time. Target delineation of boost treatment was done by computed tomography (CT) scan of tumor bearing area and high resolution ultrasonography (USG). EBRT was immediately followed by local boost at the primary tumor bearing site of breast with 8 to12 MeV electron beam to a dose of 15 Gy in 6 fractions.
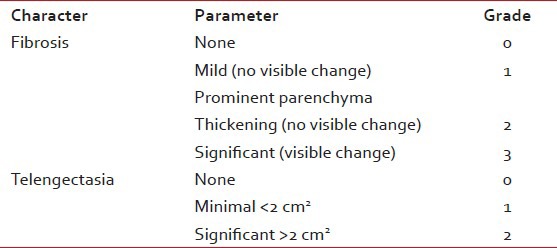
Arm-B patients were undergone target delineation and boost immediately with HDR 192Ir interstitial brachytherapy to a dose of 15 Gy in 3 fractions at 6 h apart. Rigid catheters were used for interstitial implant. Before commencing radiation, the implant geometry was verified with orthogonal simulation films and the dosimetric optimization was performed using treatment planning system. The basal dose rate was calculated at the inter source position followed by dose normalization to these points. The dose was then prescribed to the 85% referral isodose curve. During radiotherapy, patients were examined at least once a week to note any untoward effect of therapy. If any such complaint was present it was graded according to the Radiation Therapy Oncology Group (RTOG) common toxicity criteria for acute toxicities version 2.[30] Estimation of hemoglobin level and total leukocyte count was done weekly. Attempts were made to maintain a high performance status of the patient. Patients were evaluated at 2 weeks and 6 weeks after the completion of radiotherapy to look for the effects of radiation. Subsequently they were followed-up at every 3 months interval. At each visit a complete medical history was obtained and patients were asked about both solicited and unsolicited adverse effects. During the follow-up visits, factors assessed were local control, disease free survival (DFS) and cosmesis. Apart from detail clinical examinations, a post-treatment mammogram was obtained 1 year after the initial mammogram and at least 6 months after completion of radiation therapy. Thereafter, unless otherwise indicated, a yearly mammographic evaluation was performed (ASCO 2006 update). Cosmesis was assessed objectively by the treating physician and subjectively by the patient. The overall cosmetic outcome was scored according to the scale which is shown in a tabular form[31,32] [Table 1]. Correlation was made for both the arms and strata with months of DFS and level of cosmesis achieved using Chi-square test, Fisher's exact test and Student's t-test as appropriate. There were two ways used to compute a P value from a contingency table. Fisher's test was the best choice as it always gives the exact P value, whereas the Chi-square test only calculates an approximate P value. The Chi-square test was required to calculate for more than two contingency data. With small sample sizes, though Chi-square is not accurate, the Yates’ continuity correction was used to make the Chi-square approximation better.
Table 1
Overall cosmetic outcome scoring
RESULTS
A total of 40 patients of EBC were enrolled in the study. Electron boost was delivered to 20 patients (Arm A) after completion of EBRT and brachytherapy boost (Arm B) was given to 20 patients after completion of EBRT. There was no statistically significant difference in age distribution between the two arms (P = 0.333). The study intended to recruit patients with EBC. Majority of the patients were in Stage II (77.5%) and the rest were in Stage I (22.5%). The difference in stage distribution was not statistically significant (P = 1.000). The morphological characteristics difference in both the arms did not have statistically significant difference (P = 0.326). The most common scar pattern in both the arms was double scar, i.e., one for tumor excision and the other for axillary clearance accounting for about 80% in both the arms. The single chest wall scar was 20% (both tumor excision and axillary clearance done through one extended incision) in both the arms. The type of conservation surgery most commonly performed was lumpectomy in both the arms. Wide local excision or segmentectomy or quadrentectomy were done in a minimum number of cases. Hormone receptor status was almost similar in both arms. During EBRT all patients received whole breast and axillary irradiation on 6 MV linac. About 70% of patients of both the arms had separation less than 21 cm and the maximum separation was 26 cm (P = 0.497). USG and CT scan were helpful in 50% of cases of either arm. Among that 50%, CT scan was depictive in 20% of cases and USG was depictive 30% of cases with no statistical significance. Electron energy was used, depending on the depth of tumor cavity [Table 2]. In the breast implant cases, the number of planes implanted was commonly double planes (15), single plane was given to four patients and a single patient received triple plane, maximum number of catheters used were thirteen and their distribution were like the following table [Table 3]. The recruited patients were periodically examined and investigated for presence of any of the acute toxicities according to the RTOG toxicity criteria. The details of the grades of anemia, leucopoenia and the radiation induced dermatitis observed in both the arms which were not statistically significant. On follow up in Arm A, excellent cosmesis was observed in 80% of cases where as in Arm B the excellent cosmesis was observed in 50% of cases. The excellent cosmesis achieved with electron beam therapy in Arm A was found to be statistically significant (P = 0.025). Disease status at last follow up was not statistically significant as both the arms were equivalent. DFS was measured from the date of commencement of EBRT to the date of first detection of recurrence of disease, if any. One distant metastasis occurred in Arm A within 10 months of initiation of treatment and one distant metastasis in Arm B came out within 3 months of starting of therapy. No local recurrence was found within the follow-up tenure.
Table 2
Electron dose and energy distribution
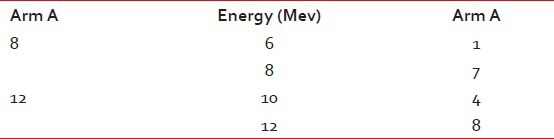
Table 3
Usage of catheters

DISCUSSION
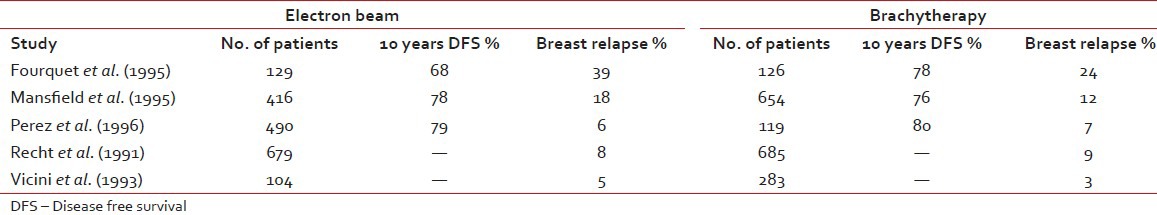
The main rationale behind tumor bed boost radiotherapy after whole breast radiotherapy of 50 Gy is that more than 60% of local recurrences occur in the tumor bed or in its vicinity which has been revealed by numerous studies.[33] This clinical observation was co-related by the pathological findings of Holland et al.[34] that residual tumor is present within 2-3 cm of the tumor bed. These findings in turn produced the concept of aggressive tumor bed radiotherapy to take care of residual local disease after BCS. Vanlimbergen et al.[37] in his study showed that tumor bed boost radiotherapy doses of 15 Gy and above decrease local recurrences by a factor of 2. This was further proved in the EORTC trial,[36] in which the patients received 50 Gy EBRT and 16 Gy boost to the tumor bed. The study showed a local recurrence rate of 2.5% at 5 years in the tumor bed boost arm. Today the American Brachytherapy Society has clearly laid down guidelines[37] that it is better to boost the tumor bed with electrons or implant in patients with either — close positive, or unknown margins, presence of extensive intraductal component (EIC) and younger patients. The EORTC trial[36] as well as the Budapest trial[38] clearly showed the benefit of tumor bed boost in improving local control rate in younger patients, particularly in those patients with age < 40 years. Nag et al.[39] in their study showed that in the absence of EIC, local control could be achieved by supplementing whole breast radiotherapy with tumor bed boost radiotherapy even in patients with positive post–op margins without submitting them to a re-excision as repeat excision would negatively influence the cosmetic outcome. These findings of Vicini are substantiated by the Hungarian trial[38] where the tumor bed boost decreased local recurrence in patients with positive post-operative margins rate from 46.7% to 8.3% respectively. Holland et al.[34] in his study described the higher risk of residual tumor around the tumor bed in patients with EIC positive tumors in comparison to patients with EIC negative tumors (74% vs. 42%). Polgar et al.[40] in his study on BCT in EIC positive patients recorded a local recurrence rate of 27.2% where the tumor bed boost radiotherapy was not given. Krishnan et al.[41] in his study showed that at 10 years, the local recurrence rates could be brought down to 9.1% by boosting the tumor bed in patients whose tumor showed EIC. Frazier et al.[43] Pezner et al.[44] and EORTC trial[36] have showed no significant differences in local control with either technique. Vanlimbergen et al.[37] in his study claims superior dosimetry and cosmesis with interstitial implants in tumor depth >28 mm. However the EORTC trial[36] showed no difference in cosmesis with electrons, photons or brachytherapy. The decisions to use either electron beam or brachytherapy boost rest on convenience, radiation safety and cost considerations. In a developing country like us where cost of primary treatment, lack of treatment resources, fear of disease recurrence and cost of treatment relapse are important impediments to wide spread use of BCT, the post-operative tumor bed implantation rather than the use of electron boost can bring down the cost factor in BCT and thereby increasing the availability of this procedure to most of the patients. On the other hand, many Institutions prefer electron beam boost due to its relative ease in set up, out-patient setting, decreased time demands. Interstitial implant can be flexible or rigid type. The rigid implants are advantageous in that they enable accurate placement of needles with appropriate spacing between individual needles in the respective planes, thereby maintaining dose homogeneity as well as preventing the emergence of hot spots or cold spots. However the rigid needles are less comfortable to the patient and need adequate analgesic cover. An important consequence of HDR boost is tumor bed fibrosis, which in spite of being moderate to severe does not significantly affect cosmesis. Surgical, radiotherapeutic and host factors may influence cosmetic outcome. Surgical factors are extent of surgical resection, reexcision, orientation and length of the scar, separate or continuous axilla-tylectomy scars, extent of axillary dissection. Radiation therapy factors are doses to the whole breast with tangential portals, gradient of dose throughout the breast tissue, fractionation and overall duration of therapy including breaks, type and dose of boost, beam energy and volume treated. Host factors include size and shape of the breast, age, race, compliance with care and hygiene, concurrent medical illnesses (hypertension, diabetes, collagen vascular disease) and intrinsic sensitivity to radiation. Breast tissue resection around 100 cm3 was associated with rates of excellent or good cosmesis, independent of breast size (P = 0.0001). Similarly, a resected skin area of greater than 20 cm2 was correlated with a lower cosmetic result (P = 0.045). Extent of axillary surgery did not significantly affect breast cosmesis. The surgeon can aid the radiation oncologist by placing radio opaque clips in the tumor bed to minimize the boost volume. Radiation factors affecting cosmesis included treatment volume (tangential breast fields only vs. three fields or more P = 0.034), whole breast dose greater than 50 Gy (P = 0.024), total dose to the tumor site greater than 65 Gy (P = 0.06). Daily fraction size of 1.8 Gy versus 2 Gy, boost versus no boost, type of boost (brachytherapy vs. electrons), total irradiation dose, chest wall or bridge separation affecting cosmetic outcome was assessed by midbridge separation versus prescribed midbridge dose and by midbridge separation versus photon energy. Delivery of conventional fractionation of 180-200c Gy per fraction minimizes reaction of the skin and breast tissue and optimizes the possibility of a good cosmetic result. Patients older than 60 years of age had lower excellent cosmetic scores compared with patients 60 years of age or younger. Tumor size significantly influenced cosmetic outcome, with 41% of patients with a tumor size of 2 cm or less having excellent cosmetic outcomes compared with 30% for tumors 2.1-5 cm in size (P = 0.05). Pezner[44] noted that fibrosis comprises cosmetic results in breast conservation therapy and it is usually related to the use of a local boost. Fourquet et al. in their study described a randomized study in which 255 patients were treated with boost irradiation and boost with reduced tangential 60Co fields or an interstitial implant. Cosmetic evaluation carried out in 120 patients showed satisfactory cosmesis in 75% of the 60Co and 71% of the 192Ir group [Tables
[Tables44–6]. The present study revealed that local recurrence was absent in both the study arms although duration of follow-up period was not so long. Regarding cosmesis, electron beam boost arm showed better cosmetic results than brachytherapy boost arm [Table 7].Table 4
Comparison of cosmetic results in BCT
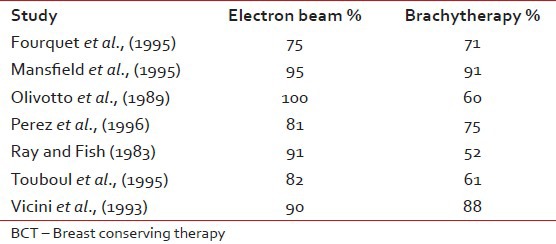
Table 6
Cosmetic results w.r.t. radiation volume, dose, and boost technique
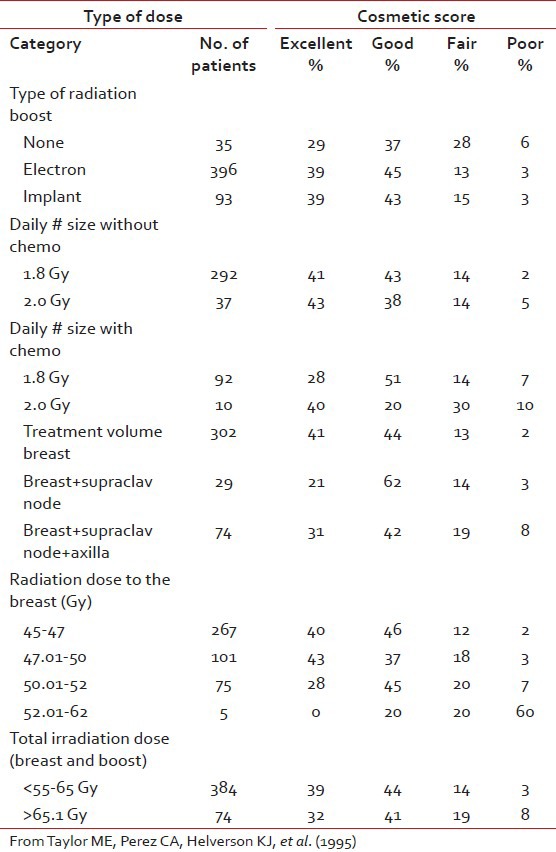
Table 7
Comparison of cosmesis in both the arms
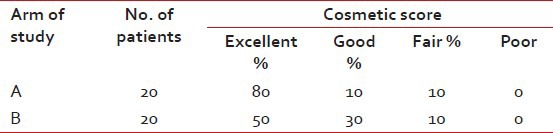
Table 5
Comparison of different studies w.r.t. relapse and DFS
To summarize, we had treated 20 patients in each arm either with electron beam boost or with brachytherapy boost after completion of EBRT. On completion of treatment, the cosmetic score was higher in electron arm and DFS, toxicity were comparable statistically in both the groups.
CONCLUSION
Based on the above comparative study on breast conservation treatment boost demonstrated good cosmetic result with electron boost and 100% local control with both the technique. However if there is a more number of patients with longer period of follow-up, we could have got the actual picture to verify our results and assess long-term survival data.
ACKNOWLEDGMENTS
We take this opportunity to thank Dr. Jahar Majumdar Specialist, Department of Surgical Oncology and Dr. Kalyan Kusum Mukherjee Consultant, Department of Medical Oncology, CNCI, Kolkata, who were equally helpful and instrumental in the preparation of this piece of work. We sincerely thank Dr. Dilip Kumar Roy, chief physicist and Radiation Safety Officer, and the other physicists in the department who also extended their support and encouragement. We also express our gratitude to all the staff of the Department of Radiotherapy as well as our institution for completion of this work. We further extend our thanks to all those patients who, at the time of their sufferings, have taught us so much.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

