Tumor Infiltrating Cytotoxic CD8 T-Cells Predict Clinical Outcome of Neuroblastoma in Children
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 159-164
DOI: DOI: 10.4103/ijmpo.ijmpo_78_17
Abstract
Context: Neuroblastoma is often infiltrated by inflammatory cells, particularly macrophages and T lymphocytes, but the significance of these cells remains unclear. One possible role of these inflammatory cells is that they represent a cell-mediated immune response against cancer. CD8+ lymphocytes are a known crucial component of cell-mediated immunity. The purpose of this study was to explore the prognostic value of tumor-infiltrating CD8+ cytotoxic lymphocytes in Neuroblastoma. Subjects and Methods:Tumor-infiltrating CD8+ lymphocytes were assessed by immunohistochemical staining of tumor tissue from 36 neuroblastoma from April 2008 to May 2015. The number of CD8+ T-cells was counted in tumor nest (intratumoral) and in the fibrovascular stroma of tumor (peritumoral), and their relationship with clinicopathologic outcome was determined. Results: The total number of CD8+ cells was inversely correlated with tumor histology grade (P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="b" xss=removed>Conclusions: In this analysis, total CD8 T-cell count was a dependent prognostic factor in children. Total number and stromal CD8 lymphocytes were associated with better patient survival (P < 0>
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context: Neuroblastoma is often infiltrated by inflammatory cells, particularly macrophages and T lymphocytes, but the significance of these cells remains unclear. One possible role of these inflammatory cells is that they represent a cell-mediated immune response against cancer. CD8+ lymphocytes are a known crucial component of cell-mediated immunity. The purpose of this study was to explore the prognostic value of tumor-infiltrating CD8+ cytotoxic lymphocytes in Neuroblastoma. Subjects and Methods:Tumor-infiltrating CD8+ lymphocytes were assessed by immunohistochemical staining of tumor tissue from 36 neuroblastoma from April 2008 to May 2015. The number of CD8+ T-cells was counted in tumor nest (intratumoral) and in the fibrovascular stroma of tumor (peritumoral), and their relationship with clinicopathologic outcome was determined. Results: The total number of CD8+ cells was inversely correlated with tumor histology grade (P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="b" xss=removed>Conclusions: In this analysis, total CD8 T-cell count was a dependent prognostic factor in children. Total number and stromal CD8 lymphocytes were associated with better patient survival (P < 0>
Introduction
Dramatic improvements in survival have been achieved for children and adolescents with cancer.[1] Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1],[2],[3] For neuroblastoma, the 5-year survival rate increased over the same time, from 86% to 95% for children younger than 1 year and from 34% to 68% for children aged 1–14 years.[2] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. Numerous innate and adaptive immune effector cells and molecules participate in the recognition and destruction of cancer cells, a process that is known as cancer immunosurveillance.[4],[5],[6] immune system's natural capacity to detect and destroy abnormal cells may prevent the development of many cancers. However, cancer cells are sometimes able to avoid detection and destruction by the immune system. Cancer cells may reduce the expression of tumor antigens on their surface, making it harder for the immune system to detect them, express proteins on their surface that induce immune cell inactivation and induce cells in the surrounding environment (microenvironment) to release substances that suppress immune responses and promote tumor cell proliferation and survival.[6] In the past few years, the rapidly advancing field of cancer immunology has produced several new methods of treating cancer, called immunotherapies that increase the strength of immune responses against tumors. Immunotherapies either stimulate the activities of specific components of the immune system or counteract signals produced by cancer cells that suppress immune responses. The present study was designed to analyze CD8+ T-cells in neuroblastoma, which showed that CD8+ T-cells infiltrated into cancer cell nests could reflect antitumor immunity. The various T-cell subsets infiltrating neuroblastoma is limited to a few studies conducted on a small number of specimens and show different types of immune cells infiltrating neuroblastoma. Some authors identified populations of CD4+ and CD8+ T-cells in NB, and others show CD25+ T-cells or cells with effector memory phenotype in NB.[7],[8] However, it is still unclear whether the presence of CD8+ cytotoxic lymphocytes provides any prognostic information in childhood neuroblastoma. Therefore, the aim was to analyze the influence of density and distribution of CD8+ cytotoxic lymphocytes on patient prognosis in well-characterized series of children with neuroblastoma during about 5 years follow-up.
Subjects and Methods
We retrospectively analyzed 36 children with neuroblastoma in Iran medical Science University (Ali Asghar hospital) between April 2008 and May 2015. The minimum follow-up period for every individual case was set to be 18 months. Informed consent was obtained from every patient's parent to be involved in the study. We have excluded the patients with immunosuppressive problems or who suffered from chronic background disease. None of these patients received preoperative immunotherapy. The age of patients ranged from 2 to 108 months of age (mean, 43 months). They were 22 males (61%) and 14 females (39%) and male: female ratio was 1.6. The patients received complete resection of the tumor with regional lymph node dissection. Resected specimens were fixed in formalin and embedded in paraffin for the routine histopathological diagnosis. Data included demographic parameters: age, gender, and also histopathology data: tumor size, histology pattern, mitosis-karyorrhexis index (MKI) index, capsular invasion, vascular invasion, necrosis, calcification, stage and finally, N-myc amplification of each tumor were extracted of files. Disease-free survival (DFS) was defined as the time interval from the date of diagnosis to the date of first relapse/progression, or the date of the last follow-up for surviving patients.
Immunohistochemistry
Formaldehyde-fixed paraffin-embedded blocks were cut into 6 ?m sections for immunohistochemical staining. At first, samples were deparaffinized and subjected to heat-induced antigen retrieval using EnVision FLEX Target retrieval solution at low- or high-pH (citrate buffer pH 6.1 and Tris/EDTA pH 9.0, respectively) at 96°C for 15 min with PT-link (Dako). For single staining, the avidin/biotin blocking system (Thermo Fisher Scientific, Fremont, CA, USA) was used according to the manufacturer's instructions (DakoCytomation, Glostrup, Denmark, USA). Tissue sections were incubated (60 min at room temperature) with monoclonal antibodies against CD8 (clone C8/144B, dilution 1:100) followed by incubation with streptavidin-alkaline phosphatase (Dako). Bound streptavidin was detected with Fast Red chromogen substrate (Dako) and levamisole in the reaction mixture for 10 min at room temperature. Sections of normal tonsils were used as positive controls of immunohistochemistry staining of CD8+ lymphocytes which are distributed mainly in the paracortical lymphoid tissue of tonsil.
Manual and digital microscope acquisition
The digital microscope used for real-time slide browsing and workflow control from remote workstations. Furthermore, two pathologists counted total numbers of CD8+ T lymphocytes each tumor core in two compartments: in nest and peritumoral fibrovascular stroma using a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan). The density of CD8 lymphocyte in the peritumoral fibrovascular stroma (peritumoral location) and in nests of tumoral cells (n est location) were recorded by two-blinded examiners as the number of positive cells per unit tissue surface area (mm 2) which is defined the density of tumor cells. For statistical analysis, the logarithm of the mean density of three fields for each sample was used. We semiquantitatively scored the degrees of infiltration into four groups as follows: 0, nil; I, mild; II, moderate; and III, severe. CD8+ T-cells were counted in two compartments in each tumor: in nest location and in peritumoral location. The total number of CD8+ T-cells was determined by combining the counts for the two locations. The average numbers of 0, 1–19, 20–49, and over 50 were scored as 0, I, II, and III, respectively. Scores were also rechecked randomly by a second observer. Interobserver agreement was found (? = 0.69).
Statistical analysis
We quantified or semi-quantified each variable as described above and then made correlation with the patients' clinical outcome with Pearson's Chi-squared method for each variable and using computer Software SPSS Version 16.0 (Chicago, SPSS Inc). We judged correlation significant with Fisher's exact test, crosstab, one-way ANOVA, and then reported P value for correlation effects of each variable.
Results
We retrospectively analyzed 36 children with neuroblastoma to investigate the relationship between the type, density, and location of CD8 T-cells within neuroblastoma lesions and the clinical outcome of patients; we performed in situ immunohistochemical analysis in 36 neuroblastoma samples about 7-year follow-up data. The age of patients ranged from 5 to 108 months of age (mean, 43 months). They were 22 males (61%) and 14 females (39%) and the male: female ratio was 1.6. The density of total T lymphocytes (CD8+) quantified in tumor cell nests and peritumoral fibrovascular stroma ranged from samples with prominent infiltrates in cohort study, to others with no infiltration [Figure 1]a,[Figure 1]b,[Figure 1]c.
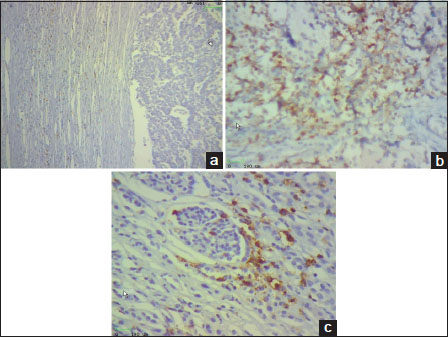
| Figure.1
Representative density of CD8+ T-cells in neuroblastoma samples. CD8+ T-cells (brown) and tumor cells (blue) are shown in septa (1a) and in nest (1b) and perivascular regions (1c). The density of CD8+ T-cells was recorded as the number of positive cells per unit of tissue surface area (Original magnification ×200) [Figure 1]a,[Figure 1]b,[Figure 1]c
DFS, from the date of diagnosis to the date of first relapse/progression, or the date of the last follow-up was performed by stratifying the subjects according to score value for CD8+ T-cell density [Figure 2]a and [Figure 2]b. The density of CD8+ T-cells in both locations was significantly correlated with patient outcome.
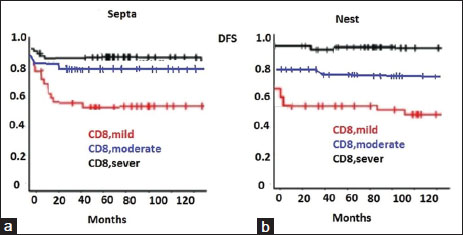
| Figure.2
Kaplan–Meier curves show the DFS of patients according to the CD8+ T-cell density scale in the septa (2a) and nest (2b) tumor regions [Figure 2]a and [Figure 2]b.
We found that total density of CD8+ cells in two location, intratumoral nest, and peritumoral fibrovascular stroma were inversely correlated with tumor histology degree (P < 0>
|
Patient |
Tumor Stage |
Tumor Grade |
IL (nest) |
IL (stroma) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
O |
I |
II |
III |
O |
I |
II |
III |
|||
|
1 |
II |
Favorable |
+ |
+ |
||||||
|
2 |
II |
Favorable |
+ |
+ |
||||||
|
3 |
II |
unFavorable |
+ |
+ |
||||||
|
4 |
III |
Favorable |
+ |
+ |
||||||
|
5 |
II |
Favorable |
+ |
+ |
||||||
|
6 |
II |
Favorable |
+ |
+ |
||||||
|
7 |
I |
Favorable |
+ |
+ |
||||||
|
8 |
IV |
unFavorable |
+ |
+ |
||||||
|
9 |
II |
Favorable |
+ |
+ |
||||||
|
10 |
I |
Favorable |
+ |
+ |
||||||
|
11 |
II |
Favorable |
+ |
+ |
||||||
|
12 |
II |
Favorable |
+ |
+ |
||||||
|
13 |
III |
Favorable |
+ |
+ |
||||||
|
14 |
II |
Favorable |
+ |
+ |
||||||
|
15 |
III |
Favorable |
+ |
+ |
||||||
|
16 |
II |
Favorable |
+ |
+ |
||||||
|
17 |
I |
Favorable |
+ |
+ |
||||||
|
18 |
II |
Favorable |
+ |
+ |
||||||
|
19 |
IV |
unFavorable |
+ |
+ |
||||||
|
20 |
I |
Favorable |
+ |
+ |
||||||
|
21 |
II |
Favorable |
+ |
+ |
||||||
|
22 |
III |
unFavorable |
||||||||
|
23 |
I |
Favorable |
+ |
+ |
||||||
|
24 |
I |
Favorable |
+ |
+ |
||||||
|
25 |
IV |
unFavorable |
+ |
+ |
||||||
|
26 |
III |
unFavorable |
+ |
+ |
||||||
|
27 |
II |
Favorable |
+ |
+ |
||||||
|
28 |
II |
Favorable |
+ |
+ |
||||||
|
29 |
I |
Favorable |
+ |
+ |
||||||
|
30 |
III |
unFavorable |
+ |
+ |
||||||
|
31 |
I |
Favorable |
+ |
+ |
||||||
|
32 |
IV |
unFavorable |
+ |
+ |
||||||
|
33 |
II |
Favorable |
+ |
+ |
||||||
|
34 |
III |
Favorable |
+ |
+ |
||||||
|
35 |
I |
Favorable |
+ |
+ |
||||||
|
36 |
II |
Favorable |
+ |
+ |
||||||
|
Tumor Stage |
P value<0> |
|||||||||
|
Tumor Grade |
P value<0> |
|||||||||
|
Characteristics |
%Patients |
iTIL mean score |
sTIL mean score |
P |
|---|---|---|---|---|
|
Age at diagnosis, months |
0.097 |
|||
|
< 18> |
12 (33.5%) |
1 |
1 |
|
|
?18 months and <5> |
18 (50%) |
3 |
2 |
|
|
?5 years Sex |
6 (16.5%) |
2 |
1 |
0.142 |
|
Male |
22 (61%) |
2 |
2 |
|
|
Female |
||||
|
Tumor size (cm) |
14 (39%) |
2 |
1 |
0.722 |
|
?2 |
5 (14%) |
2 |
1 |
|
|
>2-5 |
16 (44.5%) |
3 |
2 |
|
|
>5 |
15 (41.5%) |
2 |
2 |
|
|
Grade (INPC) |
0.001 |
|||
|
Favorable Histopathology |
28 (77.5%) |
2 |
1 |
|
|
Unfavorable Histopathology Nodal status |
8 (22.5%) |
1 |
2 |
0.002 |
|
Positive |
11 (30%) |
1 |
2 |
|
|
Negative |
||||
|
Lymph vascular invasion |
25 (70%) |
3 |
2 |
0.005 |
|
Present |
22 (61%) |
1 |
2 |
|
|
Not present Capsular invasion |
14 (39%) |
3 |
2 |
0.003 |
|
Present |
11 (30%) |
1 |
1 |
|
|
Not present Tumoral Necrosis |
25 (70%) |
3 |
2 |
0.001 |
|
Present |
10 (27.5%) |
1 |
1 |
|
|
Not present Distant Metastasis |
26 (72.5%) |
3 |
2 |
0.002 |
|
Present |
4 (11%) |
1 |
1 |
|
|
Not present Stage (INSS) |
32 (89%) |
3 |
2 |
0.003 |
|
I |
9 (25%) |
3 |
2 |
|
|
II |
16 (44.5%) |
2 |
2 |
|
|
III |
7 (19.5%) |
1 |
1 |
|
|
IV |
||||
|
N-myc oncogene |
4 (11%) |
0 |
1 |
0.002 |
|
Present |
30 (83%) |
1 |
2 |
|
|
Not present Tumor Regression |
6 (17%) |
3 |
1 |
0.001 |
|
Present |
4 (11%) |
0 |
1 |
|
|
Not present |
32 (89%) |
3 |
2 |

| Figure.1

| Figure.2
References
- owlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF. et al.,(eds). SEER Cancer Statistics Review, 1975-2010, Section 29. Bethesda, MD: National Cancer Institute; 2013
- mith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer 2014; 120: 2497-506
- owlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF. et al., (eds). SEER Cancer Statistics Review, 1975-2012, Bethesda, MD: National Cancer Institute; 2014
- unn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21: 137-48
- im R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007; 121: 1-14
- emaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM. et al. Cancer and inflammation: Promise for biologic therapy. J Immunother 2010; 33: 335-51
- acchetti P, Prigione I, Ghiotto F, Tasso P, Garaventa A, Pistoia V. et al. Functional and molecular characterization of tumour-infiltrating lymphocytes and clones thereof from a major-histocompatibility-complex-negative human tumour: Neuroblastoma. Cancer Immunol Immunother 1996; 42: 170-8
- arlson LM, De GeerA, Sveinbjørnsson B, Orrego A, Martinsson T, Kogner P. et al. The microenvironment of human neuroblastoma supports the activation of tumor-associated T lymphocytes. Oncoimmunology 2013; 2: e23618
- artin RF, Beckwith JB. Lymphoid infiltrates in neuroblastomas: Their occurrence and prognostic significance. J Pediatr Surg 1968; 3: 161-4
- Squire R, Fowler CL, Brooks SP, Rich GA, Cooney DR. The relationship of class I MHC antigen expression to stage IV-S disease and survival in neuroblastoma. J Pediatr Surg 1990; 25: 381-6
- Valteau D, Scott V, Carcelain G, Hartmann O, Escudier B, Hercend T. et al. T-cell receptor repertoire in neuroblastoma patients. Cancer Res 1996; 56: 362-9
- Mina M, Boldrini R, Citti A, Romania P, D'Alicandro V, De Ioris MK. et al. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology 2015; 4: e1019981
- Fridman WH, Mlecnik B, Bindea G, Pagès F, Galon J. Immunosurveillance in human non-viral cancers. Curr Opin Immunol 2011; 23: 272-8
- Martinet L, Garrido I, Filleron T, Le GuellecS, Bellard E, Fournie JJ. et al. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71: 5678-87
- Ladoire S, Arnould L, Mignot G, Apetoh L, Rébé C, Sarin YK. et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer 2011; 105: 366-71
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH. et al. Chemokine-driven lymphocyte infiltration: An early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61: 427-38
- Lança T, Silva-Santos B. et al. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology 2012; 1: 717-25
- Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K. et al. Smaller neuroblastoma tumors in mice intratumorally treated with Hu14. 18-IL2 have more activated tumor-infiltrating lymphocytes and better outcome. Cancer Immunol Immunother 2013; 62: 1303-13
- d">19 Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233: 1318-21
- Seeger RC. Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol 2011; 21: 229-37
- Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 2014; 257: 56-71
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T. et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29: 610-8
- Angevin E, Kremer F, Gaudin C, Hercend T, Triebel F. Analysis of T-cell immune response in renal cell carcinoma: Polarization to type 1-like differentiation pattern, clonal T-cell expansion and tumor-specific cytotoxicity. Int J Cancer 1997; 72: 431-40
- Elder DE, Guerry D, VanHorn M, Hurwitz S, Zehngebot L, Goldman LI. et al. The role of lymph node dissection for clinical stage I malignant melanoma of intermediate thickness (1.51-3.99 mm). Cancer 1985; 56: 413-8
- Mihm MCJr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996; 74: 43-7
- Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A 2004; 101 Suppl 2: 14639-45
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS. et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 1994; 86: 1159-66
- Stewart TH, Heppner GH. Immunological enhancement of breast cancer. Parasitology 1997; 115 Suppl: S141-53
- Mina M, Boldrini R, Citti A, Romania P, D'Alicandro V, De Ioris MK. et al. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology 2015; 4: e1019981


 PDF
PDF  Views
Views  Share
Share

