Upfront Maintenance Poly(Adenosine Diphosphate Ribose) Polymerase Inhibitors in Ovarian Cancer: A Ray of Hope or Just a Mirage!
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 173-181
DOI: DOI: 10.4103/ijmpo.ijmpo_2_20
Abstract
Poly(adenosine diphosphate ribose) polymerase inhibitors (PARPis), when used in patients harboring tumor with homologous recombination deficiency, with or without BRCA mutation, have shown favorable outcomes in relapsed, advanced metastatic breast and ovarian cancers. Olaparib, niraparib, and rucaparib have been approved as maintenance therapy in platinum-sensitive, relapsed, high-grade epithelial ovarian cancer (EOC) responsive to platinum doublet. Olaparib and rucaparib as monotherapy are also indicated in patients who have progressed on three or more lines of chemotherapy, irrespective of platinum sensitivity, in germline or somatic BRCA 1/2-mutated, PARPi-naive patients. Recently, four large multicentric, international Phase III randomized clinical trials have reported outcomes of PARPi in first-line advanced EOC as maintenance therapy either alone or in combination with bevacizumab. Previously bevacizumab, pazopanib, nindetanib, or maintenance chemotherapy in first-line setting has resulted in modest improvements in progression free survival, albeit with significant toxicities and poor cost-effectiveness. We offer in this review to dissect the data pertaining to randomized clinical trials of PARPi use as maintenance therapy in upfront EOCs and ruminate about its role in the contemporary management of ovarian cancers.
Keywords
Epithelial ovarian cancer - maintenance therapy - poly (adenosine diphosphate ribose) polymerase inhibitorsPublication History
Received: 07 January 2020
Accepted: 02 February 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Poly(adenosine diphosphate ribose) polymerase inhibitors (PARPis), when used in patients harboring tumor with homologous recombination deficiency, with or without BRCA mutation, have shown favorable outcomes in relapsed, advanced metastatic breast and ovarian cancers. Olaparib, niraparib, and rucaparib have been approved as maintenance therapy in platinum-sensitive, relapsed, high-grade epithelial ovarian cancer (EOC) responsive to platinum doublet. Olaparib and rucaparib as monotherapy are also indicated in patients who have progressed on three or more lines of chemotherapy, irrespective of platinum sensitivity, in germline or somatic BRCA 1/2-mutated, PARPi-naive patients. Recently, four large multicentric, international Phase III randomized clinical trials have reported outcomes of PARPi in first-line advanced EOC as maintenance therapy either alone or in combination with bevacizumab. Previously bevacizumab, pazopanib, nindetanib, or maintenance chemotherapy in first-line setting has resulted in modest improvements in progression free survival, albeit with significant toxicities and poor cost-effectiveness. We offer in this review to dissect the data pertaining to randomized clinical trials of PARPi use as maintenance therapy in upfront EOCs and ruminate about its role in the contemporary management of ovarian cancers.
Keywords
Epithelial ovarian cancer - maintenance therapy - poly (adenosine diphosphate ribose) polymerase inhibitorsIntroduction
Among gynecological malignancies, epithelial ovarian cancer (EOC) is the most common cause of mortality across the world.[1] Unfortunately, more than three-fourth of ovarian cancers are diagnosed in Stages III and IV where 5-year survival is <30%.[1] The standard therapy for advanced EOC is upfront optimal cytoreductive surgery with residual disease of <1 cm followed by six cycles of three weekly paclitaxel and carboplatin chemotherapy doublet. In patients who are not candidates for primary cytoreductive surgery, neoadjuvant chemotherapy followed by interval cytoreduction is an acceptable alternative.[2] In some studies, combination of intravenous and intraperitoneal chemotherapy (IV/IP) or dose-dense intravenous chemotherapy has shown improvements in overall survival.[3],[4],[5],[6]
Despite the above advances in therapy eventually, more than 70% of patients will relapse between 6 and 24 months of first-line therapy. After brief ill-sustained response to second- and third-line therapy, most of these patients will succumb to the disease. This calls for an effective novel maintenance therapy that can sustain the excellent response achieved after first-line chemotherapy. This would lead to durable, long-term, sustained remissions, perhaps even cure. Strategies using maintenance chemotherapy, bevacizumab, or pazopanib provided only modest improvements in outcomes, but with significant adverse events and cost.[7],[8],[9],[10] Recently, poly (adenosine diphosphate ribose) polymerase inhibitors (PARPis), olaparib, veliparib and niraparib, have demonstrated benefit in delaying disease progression when added to adjuvant chemotherapy followed by oral maintenance or oral maintenance alone after completion of standard therapy in newly diagnosed high-grade serous or endometrioid EOC showing partial to complete response to first-line platinum doublet therapy, especially in patients having germline/somatic BRCA 1 and 2 mutation or homologous recombination deficiency (HRD).[11],[12],[13],[14],[15]
In this review, we attempt to summarize the eligibility, efficacy, and safety data from various Phase III randomized interventional clinical trials in first-line/de novo high-grade EOCs where PARPis have shown to have a favorable impact on outcomes as maintenance therapy. We also describe the challenges associated with germline/somatic BRCA and homologous recombination deficiency (HRD) testing, in the Indian context. We also attempt to answer the contentious question regarding the integration of PARPis in addition to or as an alternative to bevacizumab-based maintenance strategies in EOCs. The use of single-agent PARPis instead of chemotherapy in platinum-sensitive setting or as maintenance after relapse and in disease refractory to multiple lines of chemotherapy is beyond the purview of this article.
Mechanism of Action
PARP 1 and 2 play a critical role in the repair of single-strand breaks (SSBs) that occur in healthy deoxyribonucleic acid (DNA) of normal cells every day. Moreover, when a DNA replication fork encounters a SSB during mitosis, it may convert it to double-strand break (DSBs) leading to cell cycle arrest. Usually, this gets repaired in healthy individuals with proficient homologous recombination repair (HRR) pathways, hence preventing otherwise catastrophic cell death by apoptosis.[16] PARPis, by blocking critical PARP enzymes, prevent repair of SSBs and accentuate potentially lethal DSBs either spontaneously or under ionizing radiation, radiomimetic agents, or conventional chemotherapy.[17] However, this may be insufficient for tumor cell killing by itself in the presence of healthy proficient HRR pathways that repairs and reverses above DNA damage.[17],[18]
Synthetic lethality is a phenomenon, where the defect in HRR pathway due to either germline or somatic BRCA (in tumor cells) or other still poorly understood mechanisms results in HRR deficiency. This leads to genetic instability due to the accumulation of DSBs, which then goes unrepaired due to sustained PARPi. This induced tumor genetic instability by the above dual mechanism initiates tumor cell apoptosis and cell death under the principle of synthetic lethality. This concept after getting validated in vivo and in vitro experiments has passed translational research into clinical practice. Higher response rates and longer progression-free survival (PFS) have been observed with PARPsi in patients who harbor a mutation in germline or somatic BRCA gene or in certain other genes which result in HRR deficiency[19],[20],[21] [Figure 1].
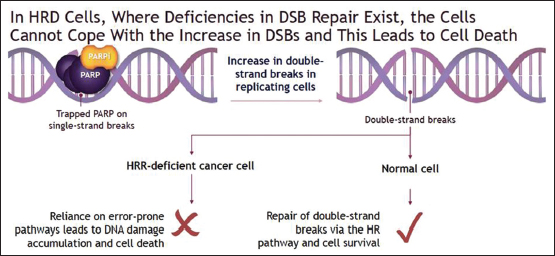
| Figure 1: Mechanism of action of poly (adenosine diphosphate ribose) polymerase inhibitors in BRCA mutation and homologous recombination deficient tumors
More than 40% of high-grade serous ovarian carcinomas are homologous recombination deficient (HRD). Of which, 14% constitute germline BRCA 1 and 2 mutations and 7% due to somatic BRCA 1 and 2 mutations. The remaining are due to less known, poorly understood deficient or defective HRR genes which confers “BRCAness” in tumors otherwise lacking classical BRCA 1 and 2 mutations[17],[18] [Figure 2]. Whatever the reason for HRR deficiency, PARPis trigger synthetic lethality, resulting in tumor-specific cell killing with relative sparing of healthy tissue, hence improving therapeutic index unlike chemotherapy or radiotherapy.
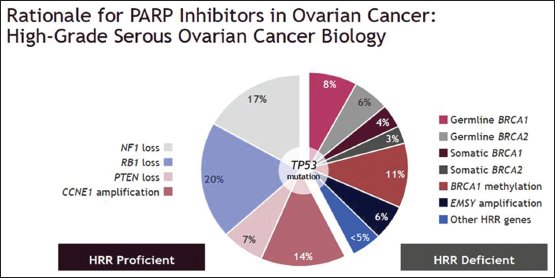
| Figure 2: Distribution of types of homologous recombination deficiency, including germline and somatic BRCA and other variants which provide BRCAness to high‑grade epithelial ovarian carcinoma
Maintenance poly (adenosine diphosphate ribose) polymerase inhibitors in first-line upfront high-grade epithelial ovarian carcinomas
Four large positive Phase III randomized clinical trials where PARPis as maintenance were used in first-line/de novo high-grade EOC were recently reported.[11],[12],[13],[14],[15] Of these, three trials had used PARPi alone, whereas the forth had compared PARPi and bevacizumab with bevacizumab alone.
Efficacy
SOLO-1 trial[11]
A multicentric, international (15 countries), Phase III randomized clinical trial, SOLO1, was conducted on women with Stage III/IV highgrade serous or endometrioid EOC who have achieved complete or partial response to firstline platinumbased doublet chemotherapy. All patients had underwent primary or interval cytoreduction surgery and were randomized in 2:1 fashion to receive tablet olaparib 300 mg twice daily or placebo. The eligibility criteria allowed women with either germline or somatic BRCA mutation, tested locally, but confirmed in the central laboratory before enrolment. However, only two patients had a somatic mutation in the olaparib arm, whereas none in the placebo. None of the women received bevacizumab as part of induction platinum doublet or maintenance.[11]
Patients in the olaparib arm who did not have any evidence of disease were allowed to stop maintenance at 2 years, whereas those having the residual disease were allowed to continue olaparib. At 3 years, freedom from disease progression or death was 60% versus 27% favoring olaparib maintenance arm (hazard ratio [HR] = 0.30 [0.23–0.41]; P = 0.001). Among secondary endpoints, freedom from second progression or death was 75% in the olaparib arm and 60% in the placebo arm (HR = 0.50 [0.32–72]; P = 0.001), despite 32% of patients on the placebo arm switched over to olaparib after the first progression. Although the overall survival data are not matured, estimated freedom from death was 84% versus 80% in olaparib and placebo arm respectively (HR = 0.95 [0.60–1.53]).[11] Time to subsequent therapy was 51 months versus 15 months favoring the olaparib arm. Health-related quality of life was not affected by olaparib maintenance compared to the placebo [Table 1].
VELIA trial[12]
In another multicentric (202 sites), international (10 countries), Phase III randomized control trial in high-grade serous EOC Stage III/IV, patients were randomized to 1:1:1 manner to (A) the control group (in which patients received chemotherapy plus placebo followed by placebo maintenance); (B) the veliparib combination-only group (in which patients received chemotherapy plus veliparib followed by placebo maintenance); or (C) the veliparib-throughout group (in which patients received chemotherapy plus veliparib followed by veliparib maintenance). The investigators enrolled 1140 patients and were powered to detect not only the primary endpoint of PFS but also the overall survival in intention-to-treat population, BRCA mutation cohort, and homologous recombination deficient cohort (including BRCA mutation). About 26% of patients had BRCA mutation, of which 19% were germline and 7% had somatic BRCA. Similarly, 29% of patients had non-BRCA homologous recombination deficiency when tested by myChoice CDx (Myriad) testing.[12]
The median PFS in BRCA mutation cohort was 34.7 months in group (C) velaparib throughout compared to 22 months in group (A) control arm (HR = 0.44 [0.28–0.68]; P = 0.001). Similarly, among homologous recombination deficient cohort and intention-to-treat population, the benefit was evident and statistically significant in the velaparib-throughout cohort compared to the placebo arm with a HR of 0.57 (0.43–0.76, P < 0.001) and 0.68 (0.56–0.83, P < 0.001), respectively, favoring velaparib maintenance. Despite higher toxicities in velaparib, the health-related quality of life was retained in the interventional arm similar to that of placebo[12] [Table 1].
PRIMA trial[13]
In another similar multicentric and international trial, PRIMA, of niraparib, patients with Stage III/IV high-grade serous epithelial and endometrioid carcinoma were randomized 2:1 to receive niraparib 300 mg OD versus placebo, till 36 months or disease progression. Patients having much a higher risk of relapse, such as those with residual disease after cytoreductive surgery, Stage IV unresectable disease, and those receiving neoadjuvant chemotherapy were included in this trial. However, all patients should have achieved complete or partial response to six to nine cycles of platinum-based doublet chemotherapy. More than 50% of patients had homologous recombination deficient tumor profile, either due to germline/somatic BRCA or without, as tested with a score above 42 on Myriad's myChoice test.[13]
The median duration of PFS was 21.9 versus 10.4 months, P = 0.001 favoring niraparib maintenance, in homologous recombination deficient tumors (including BRCA mutation). Similarly, the above benefit to a lesser extent was seen in the overall population in favor of niraparib with 13.8 versus 8.2 months, P = 0.001. However, with immature follow-up and lesser events for overall survival, estimated difference in overall survival at 24 months was not significant in either BRCA mutation cohort or in the overall population, despite patients in the placebo arm were not allowed to crossover to receive niraparib at progression.[12] Patient-reported quality of life was not affected with the use of niraparib and was sustained even with prolonged exposure to the drug compared to the placebo[13] [Table 1].
PAOLA-1 trial[14],[15]
In the PAOLA-1 trial, patients in clinical complete or partial response following platinum-based doublet (six to nine cycles) plus bevacizumab were randomized 2:1 to receive oral olaparib tablets at 300 mg twice daily for up to 2 years with bevacizumab or placebo plus bevacizumab at 15 mg/kg on day 1 every 3 weeks for 15 months, which included doses received during chemotherapy. The patients were enrolled irrespective of BRCA mutation status or HRD. MyChoice by Myriad Diagnostics was used for homologous recombination deficiency testing where HR score was more than or equal to 42 and BRCA mutation was labeled as HRD. The primary endpoint was investigator-assessed PFS. With a median follow-up of 24 months with 59% data maturity, median PFS was 22.1 versus 16.6 months in olaparib and bevacizumab combined maintenance versus bevacizumab maintenance alone with a HR of 0.59 (95% confidence interval [CI]: 0.49–0.79), P = 0.001 in the overall population.[14],[15]
The benefit was statistically significant and clinically profound for BRCA mutation and homologous recombination deficient patients (non-BRCA) with a HR of 0.31 and 0.43, respectively, but this was insignificant for patients with negative or unknown homologous recombination deficiency (HR: 0.92). The data regarding PFS2 and overall survival are not matured, yet to be reported[14],[15] [Table 1].
Safety
SOLO-1 trial[11]
In SOLO-1 trial of olaparib maintenance, any Grade 3/4 toxicity was more common in the olaparib arm (39%) compared to the placebo (18%). The most common among them was anemia in 22% of patients in the olaparib arm compared to 2% in the placebo. Serious adverse events occurred in 21% of patients in the olaparib arm. Treatment discontinuation (12% versus 2%), dose reduction (28% versus 3%), and interruptions (52% versus 17%) were more frequent in the olaparib arm, attributed primarily to anemia and nausea. Acute myeloid leukemia was seen in three patients receiving olaparib compared to none in the placebo arm, all three cases happened well after completion of olaparib therapy, suggesting a need for continued surveillance.[11]
VELIA trial[12]
In VELIA trial of velaparib, the most common adverse events were nausea in 80% of patients, but 90% of that was Grade 1 and 2 only. Dose reductions (6% versus 2%) and interruptions (58% and 39%) were higher in the velaparib-throughout group compared to the placebo. Drug discontinuation was 19% in the velaparib maintenance compared to 6% in the control arm, mainly attributed to nausea. Grade 3/4 thrombocytopenia, anemia, and neutropenia were significantly worse in the velaparib maintenance/throughout group with 28%, 38%, and 58%, respectively. One patient each of myelodysplastic syndrome and acute myeloid leukemia was seen in the velaparib group, both having BRCA mutation, compared to none in the placebo arm.[12]
PRIMA trial[13]
Dose reduction and interruptions were seen in 70% and 80% of patients receiving niraparib in the PRIMA trial, respectively. However, adverse event-related drug discontinuation was seen in only 12% in the niraparib arm. Grade 3/4 hematological toxicities were higher with niraparib use, with 31% anemia, 29% thrombocytopenia, and 13% neutropenia. Hematological toxicities were the major reason for drug interruptions, dose reductions, and discontinuation. Unlike other trials such as SOLO-1, VELIA, SOLO-2, and ARIEL, only one patient had developed myelodysplastic syndrome in PRIMA trial with niraparib.[13]
PAOLA-1 trial[14],[15]
In PAOLA-1 trial, Grade 3–4 adverse events were similar across the olaparib and bevacizumab combined maintenance arm and bevacizumab-alone arm, 57% versus 51%, respectively. Anemia was significantly higher in olaparib combined maintenance, 17% versus 1%, whereas hypertension was higher in the bevacizumab-alone arm, 19% versus 30%. Treatment discontinuation was 20% in the combination arm compared to 6% in the bevacizumab-only maintenance. Dose interruptions and dose reductions were higher in the combined maintenance arm, 54% versus 24% and 41% versus 7%, respectively. Despite the above toxicity profile, the health-related quality of life was not different in either arm.[14],[15]
Discussion
SOLO-1 trial[11]
The results of SOLO-1 trial demonstrated a 70% reduction in risk of disease progression or death in patients having BRCA 1/2 mutation and receiving olaparib maintenance for 2 years after completion of standard induction platinum-based doublet chemotherapy and cytoreductive surgery. Compared to olaparib use in platinum-sensitive (>6 months), relapsed, ovarian cancers in SOLO-2 trial, use of olaparib as upfront maintenance showed longer PFS advantage.[22] Upfront maintenance with olaparib should be preferred over its use in the relapsed setting where the magnitude of benefit is lesser in all outcome endpoints measured.[11],[22] As the time to subsequent treatment was delayed with olaparib maintenance upfront, patients benefitted from longer chemotherapy-free interval, extending more than 2 years, before the subsequent line of chemotherapy was started. Despite 32% crossover to olaparib, second PFS still favored olaparib maintenance, suggesting lack of detrimental effect of its early use on efficacy of subsequent line of therapy.[11]
After stopping olaparib at 2 years, the survival curve remained stable and apart suggesting extended benefit at the 3rd year. Hence, olaparib in small subset of patients, by delaying progression, is converting EOC into a slow chronic disease. However, caution and vigilance are merited to detect myelodysplastic syndrome and acute myeloid leukemia on longer follow-up.[11] The result of this trial is applicable exclusively for germline BRCA-mutated patients achieving complete or partial response to initial chemotherapy, as those with homologous recombination deficiency were not tested or enrolled and <1% had somatic BRCA mutation in the intervention arm. However, longer follow-up for overall survival and late toxicities are merited.
VELIA trial[12]
The VELIA trial differed from SOLO 1, in that patients started veliparib concurrently with chemotherapy and were enrolled irrespective of BRCA mutation and HRD status. The PFS was defined from the start of induction chemotherapy, rather than the start of maintenance. The trial demonstrated benefit of velaparib maintenance in BRCA-mutated and homologous recombination deficient (HRD) cohort. In patients devoid of the above biomarker selective attributes, the benefit was less profound (the upper limit of 95% CI exceeded 1 in HRD-negative cohort with a HR of 0.81). The benefit was also seen irrespective of response to combination chemotherapy, including those with a significant residual disease where cytoreductive surgery was not feasible.[12]
In the absence of pure maintenance-only arm as the comparator, the incremental benefit of using veliparib right from the beginning of chemotherapy cannot be determined. The combination-only velaparib arm (without maintenance) compared to the placebo was no better in terms of PFS with a HR of 1.22 and 1.10 in BRCA mutation and HRD, respectively.[12] This suggests that in the velaparib-throughout arm, the majority of benefit was due to velaparib maintenance after completion of chemotherapy. Further, based on cross-trial comparison of HR with PAOLA-1 AND PRIMA trial [Table 1], it is unlikely that the concurrent phase might have added any benefit. However, the trial was not designed to answer this question and the approval of velaparib is with combination chemotherapy and maintenance only. Similar to that seen in the SOLO-1 trial, the benefit of velaparib was sustained in the BRCA mutation cohort beyond 30 months of maintenance therapy.
PRIMA trial[13]
The PRIMA trial of niraparib demonstrated benefit in PFS not only in BRCA-mutated, homologous recombination deficient tumors but also in homologous recombination-proficient tumors. This makes BRCA and HRD testing partially redundant as a biomarker for the selection of patients for niraparib. As this trial demonstrated unequivocal benefit in otherwise unlikely HR-proficient tumors, this further poses a question of whether niraparib has action beyond DNA damage repair pathway defect.[13] However, to explore depths of its action in tumors devoid of HRD is beyond the scope of this review.
Despite the trial had only high-risk patients such as those with inoperable Stage III, cytoreductive surgery with gross residual disease, or Stage IV tumors, the HR with niraparib maintenance for BRCA-mutated and HRD cohorts was similar to that in SOLO-1 (olaparib) and VELIA (velaparib) trials, respectively. Moreover, compared to other first-line maintenance PARPi trials, velaparib was used for the longest duration of 36 months.[13] However, with a median follow-up of 13.8 months only, it will be worthwhile to see whether the benefit is sustained beyond 3 years of maintenance.
PAOLA-1 trial[14],[15]
The PAOLA-1 trial is the only randomized Phase III trial, yet which has shown a significant benefit of combined olaparib and bevacizumab maintenance compared to bevacizumab alone in advanced EOC. Compared to other first-line maintenance trials of olaparib alone (SOLO-1), this study had recruited patients irrespective of surgical outcomes and BRCA/HRD status.[14] However, the benefit was restricted to patients with BRCA mutation and the HRD cohort exclusively. Compared to the other first-line maintenance PARPi trials discussed above, PAOLA-1 had the highest discontinuation rate of 20% in the combined maintenance arm.[15]
Unlike SOLO-1 where curves of PFS remain separated even after 2 years of maintenance with median not reached in the olaparib arm, in PAOLA-1 trial at the 3rd year, the combination maintenance arm curve touched bevacizumab-alone curve. This suggests that the benefit of combined olaparib and bevacizumab 2-year maintenance is not durable enough in the unselected population. However, in subset of BRCA-mutated patients, the median PFS in PAOLA-1 trial exceeded 3 years, similar to that seen in SOLO-1 trial. Data from both above trials do suggest that at least in BRCA mutation cohort, 2 years maintenance with olaparib or olaparib and bevacizumab dose have durable benefit extending beyond 2 years.[11],[14],[15]
SOLO-1 trial had demonstrated the benefit of olaparib maintenance alone in germline BRCA mutation-positive tumors only, as this was the sole inclusion criteria (only 1% had somatic BRCA mutation).[11] The question was whether olaparib can extend benefit in HRD, non-BRCA, and HR-proficient/unknown tumors as well. With PAOLA-1, this debate gets settled with benefit extended apart from BRCA-mutated EOC, in HRD (non-BRCA) patients too with olaparib, albeit in combination with bevacizumab. The addition of olaparib in homologous recombination proficient/unknown patients failed to have any benefit over bevacizumab alone. Taking SOLO-1 and PAOLA 1 trials into perspective, BRCA mutation testing is a must for olaparib-alone maintenance, whereas BRCA and HRD testing should be used as a biomarker to select patients for combined olaparib and bevacizumab maintenance.[11],[14],[15]
Poly (adenosine diphosphate ribose) polymerase inhibitors, antivascular endothelial growth factor, or both as maintenance: How to select the best?
Use of bevacizumab has demonstrated modest but significant benefit in PFS in high-grade, Stage III/IV EOC when used in combination with platinum doublet and as subsequent maintenance therapy. This has been explored in two Phase III randomized clinical trials, GOG 218 and ICON 7, each using bevacizumab 15 mg/kg and 7.5 mg/kg every three weekly, for 15 and 12 months, respectively. In both trials, patients had to undergo upfront cytoreductive surgery and then receive subsequent adjuvant bevacizumab chemotherapy and maintenance bevacizumab.[7],[8] Hence, these data are not applicable in patients who had to receive neoadjuvant chemotherapy due to inoperable Stage III, bulky Stage IV, and patients with gross ascites or pleural effusion.[7],[8]
ICON7 and GOG 218 both in post hoc subset analysis showed small benefit in overall survival to high risk, Stage IV patients with gross ascites/pleural effusion, no prospective trials have shown overall survival benefit of bevacizumab as maintenance therapy yet.[9] Bevacizumab maintenance was associated with Grade 3/4 hypertension, higher rates of gastrointestinal perforation, fistula, hemorrhage, and proteinuria.[7],[8],[9] Moreover, there is a lack of cost-effectiveness of bevacizumab when used as maintenance in first-line EOC due to no overall survival benefit, cost, and higher toxicities.[7],[8],[9],[23] Two other oral anti-VEGF inhibitors, pazopanib and nindetanib, have also demonstrated improved PFS as maintenance therapy without overall survival benefit, albeit with higher Grade 3/4 toxicities.[10],[24]
In all four major maintenance anti-VEGF trials in first-line EOCs, namely GOG-218, ICON-7, AGO-OVAR-16 (Pazopanib), and AGO-OVAR-12 (nindetanib) trial, the HR for PFS was between 0.72 and 0.84 with upper limit of 95% CI between 0.82 and 0.98.[8],[9],[10],[24] Compared to this, use of PARPis as first-line maintenance in BRCA mutation-positive and homologous recombination deficient cohort (non-BRCA) gave a HR of 0.30–0.44 and 0.43–0.57 with upper limit of 95% CI of 0.41–0.68 and 0.59–0.76, respectively.[11],[12],[13],[14] This cross trial comparison, though indirect, shows the superiority of PARPis over bevacizumab maintenance alone, with 70% and 50% decrease in risk of disease progression in BRCA mutation and HRD (non-BRCA) cohorts, respectively.
BRCA mutation also confers a better independent prognostic value in terms of improved PFS and overall survival in EOC.[25] However, neither bevacizumab nor pazopanib maintenance in BRCA mutation-positive high-grade EOC confers any meaningful benefit in PFS over placebo in GOG 218 and AGO-OVAR 16 trials, respectively.[25],[26] Hence making antiVEGF alone, a less favorable option for BRCA mutation positive patients as a choice for maintenance therapy compared to PARP inhibitors. With results of VELIA, PRIMA, and PAOLA-1, even in HRD patients, PARPi alone or in combination with bevacizumab is a better choice than bevacizumab-alone maintenance.[12],[13],[14],[15] The choice between bevacizumab maintenance versus PAPRi alone or in combination with anti-VEGFR in HR-proficient/unknown tumors is still debatable. Except for niraparib (PRIMA trial), no other PARPi has demonstrated unequivocal benefit in PFS in the above cohort.[13] HR-proficient/unknown patients were not included in the SOLO-1 trial, whereas velaparib (VELIA) and combined olaparib and bevacizumab maintenance in them failed to improve outcomes compared to placebo and bevacizumab-alone maintenance, respectively.[11],[12],[14],[15]
PAOLA-1, as discussed above, has demonstrated improved PFS in BRCA mutation and HRD patients, when olaparib is used in combination with bevacizumab versus bevacizumab alone maintenance.[14],[15] The HRs for the extent of benefit among BRCA mutation patients were similar whether olaparib alone was used or was given in combination with bevacizumab, in SOLO-1 and PAOLO-1 trial, respectively, close to 0.31 [Table 1].[11],[14],[15] This questions the added benefit of bevacizumab over olaparib, at least in BRCA mutation cohort. It would have been more interesting to witness the comparison of olaparib alone versus bevacizumab alone or olaparib plus bevacizumab versus olaparib alone in BRCA mutation-positive, homologous recombination deficient or proficient tumors. This would have settled the debate; however, till then, the question of incremental benefit of adding bevacizumab over PARPi or either of them alone remains unanswered.
The PFS with bevacizumab in the BRCA nonmutated HR-proficient cohort (16 months) is not very different from the bevacizumab arm of the BRCA and HRD cohorts (17.7 months and 16 months, respectively). This would suggest that Bev is efficacious in HR-proficient BRCA nonmutant tumors where one would expect poorer PFS compared to gBRCA mutant population. However, the recent analysis of the GOG trial showed that addition of Bev did not improve outcomes in the BRCA nonmutant cohort.[25] Hence, the role of bevacizumab in HR-proficient BRCA nonmutant patients remains controversial.
BRCA mutation and homologous recombination deficiency: What is the status in India?
In a preliminary report from a prospective cross-sectional study involving patients with ovarian, peritoneal, and fallopian tube cancer from 12 different geographical sites in India, the prevalence of BRCA 1 and 2 mutation was around 22%, which is much higher compared to Western literature of 12%.[27] In a much larger study of 1000 patients done among breast and ovarian cancer patients, 30% of them had BRCA mutations.[28] Important here is to acknowledge that more sensitive multigene next-generation sequencing was used in both the above studies, rather than sequential independent gene testing.[29] Interesting to note is that one-sixth of these BRCA mutations were novel and different from that seen in the Western population and Ashkenazi Jews.[28],[30] Another smaller study done in thirty patients with exclusive EOCs found that the three classical founder mutations representative of BRCA 1 and 2 mutation were absent in the above cases and all mutations detected were novel variants of BRCA 1 and 2.[31] This confers that landscape for BRCA mutation profiling in EOC among Indians may have different and novel variants compared to the Western population.
Homologous recombination deficiency was tested in the central lab by MyChoiceCDX (Myriad diagnostics) in VELIA, PRIMA, and PAOLA-1 trials, which is now an approved companion diagnostic tool for PARPi use as maintenance therapy for HRD patients.[12],[13],[14],[15] MyChoice CDX is the next-generation sequencing in vitro diagnostic test done on extracted DNA from formalin-fixed paraffin-embedded tumor blocks which determine genetic instability score on an algorithmic scale of 1–100 through measurement of Loss of heterozygosity, Telomeric Allelic Imbalances, Large scale state transitions.[32],[33] A score of 42 and above is considered to be homologous recombination deficient cutoff and used as a selection criterion for PARPi maintenance therapy.
Similarly, in olaparib maintenance trials (SOLO-1 and PAOLA-1), BRCAAnalysisCDX (Myriad Diagnostics) was used to confirm local BRCA testing before olaparib maintenance. Both these offshore companion diagnostic costs more than 3300 United States Dollars (USD) with a turnaround time of 4 weeks. None of the government or commercial labs in India yet perform or report homologous recombination deficiency on next-generation sequencing platforms. Few commercial labs do perform BRCA testing in India with cost as less as 380 USD, but whether to use PARPi based on these reports exclusively is debatable. It is advisable to confirm BRCA local test results with centralized BRCAAnalysisCDX (Myriad Diagnostics) before starting patients on PARPi. Niraparib (PRIMA trial) is the only PAPRi in first-line maintenance, where significant benefit exists irrespective of BRCA and HRD status, hence obviating the absolute need of these tests as biomarkers for patient selection.[13] However, the extent of benefit is more profound for BRCA mutation and HRD patients even with niraparib.
Conclusion
PARP inhibitors significantly reduce the risk of disease progression in BRCA-mutated and homologous recombination deficient patients suffering from advanced EOC, who had favorable response to platinum-based doublet chemotherapy, when used as upfront maintenance therapy. The results are consistently favorable at least for those with HRD/BRCA mutant tumors across trials. Hence, testing for both biomarkers is strongly advisable. However, testing, especially for HRD, remains a challenge with varying tests and cutoffs. It should be made widely available for more patients to benefit from maintenance. Unanswered questions remain which include incremental benefit of bevacizumab, duration of maintenance, role in nonmutant/HR-proficient group where currently benefit seems to be modest. It will be interesting to see whether PFS benefits will translate to overall survival improvements with longer follow-up.
Conflict of Interest
There are no conflicts of interest.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87-108
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363: 943-53
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA. et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950-5
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S. et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34-43
- Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S. et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2014; 15: 396-405
- Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E. et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol 2013; 14: 1020-6
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365: 2473-83
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G. et al. Aphase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365: 2484-96
- Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E. et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol 2015; 16: 928-36
- du Bois A, Floquet A, Kim JW, Rau J, del Campo JM, Friedlander M. et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014; 32: 3374-82
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379: 2495-505
- Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M. et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 2019; 381: 2403-15
- González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR. et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019; 381: 2391-402
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martin A, Sevelda P. et al. Phase III PAOLA-1/ENGOT-ov25: maintenance olaparib with bevacizumab in patients with newly diagnosed, advanced ovarian cancer treated with platinum-based chemotherapy and bevacizumab as standard of care. Ann Oncol 2017; 30(Suppl 5): v851-934
- Phase III PAOLA-1/ENGOT-ov25 trial: Olaparib Plus Bevacizumab as Maintenance Therapy in Patients with Newly Diagnosed, Advanced Ovarian Cancer Treated with Platinum-based Chemotherapy Plus Bevacizumab. https://oncologypro.esmo.org/Meeting-Resources/ESMO-2019-Congress/Phase-III-PAOLA-1-ENGOT-ov25-trial-Olaparib-plus-bevacizumab-bev-as-maintenance-therapy-in-patients-pts-with-newly-diagnosed-advanced-ovarian-cancer-OC-treated-with-platinum-based-chemotherapy-PCh-plus-bev Available from: [Last accessed on 2020 Jan 05]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M. et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009; 361: 123-34
- O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015; 60: 547-60
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S. et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition. Cancer Res 2006; 66: 8109-15
- Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008; 105: 17079-84
- Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244-50
- Matulonis UA, Penson RT, Domchek SM, Kaufman B, Shapira-Frommer R, Audeh MW. et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety. Ann Oncol 2016; 27: 1013-9
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274-84
- Hinde S, Epstein D, Cook A, Embleton A, Perren T, Sculpher M. et al. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health 2016; 19: 431-9
- du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N. et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2016; 17: 78-89
- Norquist BS, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner SI. et al. Mutations in homologous recombination genes and response to treatment in GOG 218: An NRG oncology study. Gynecol Oncol 2016; 141: 2
- Harter P, Johnson T, Berton-Rigaud D, Park SY, Friedlander M, Del Campo JM. et al. BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol Oncol 2016; 140: 443-9
- Rohit K, Gupta S, Rajappa SJ, Advani SH, Agarwal A, Aggarwal S. et al. A non-interventional, multicenter study to assess prevalence of BRCA1 and BRCA2 mutation among ovarian, primary peritoneal, and fallopian tube cancer patients in India. J Clin Oncol 2019; 37 (Suppl. 15) e13073
- Singh J, Thota N, Singh S, Padhi S, Mohan P, Deshwal S. et al. Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: Prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 2018; 170: 189-96
- Mannan AU, Singh J, Lakshmikeshava R, Thota N, Singh S, Sowmya TS. et al. Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: Implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet 2016; 61: 515-22
- Valarmathi MT, Sawhney M, Deo SS, Shukla NK, Das SN. Novel germline mutations in the BRCA1 and BRCA2 genes in Indian breast and breast-ovarian cancer families. Hum Mutat 2004; 23: 205
- Sharma S, Rajaram S, Sharma T, Goel N, Agarwal S, Banerjee BD. et al. Role of BRCA1 and BRCA2 gene mutations in epithelial ovarian cancer in Indian population: A pilot study. Int J Biochem Mol Biol 2014; 5: 1-0
- Matthew WK, Becker M, Neff C, Abkevich V, Jones JT, Hou X, Wang Y. et al. Use of homologous recombination deficiency (HRD) score to enrich for niraparib sensitive high grade ovarian tumors. J Clin Oncol 2015; 33 (Suppl. 15) 5532
- Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 2016; 22: 3764-73
Address for correspondence
Publication History
Received: 07 January 2020
Accepted: 02 February 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Mechanism of action of poly (adenosine diphosphate ribose) polymerase inhibitors in BRCA mutation and homologous recombination deficient tumors

| Figure 2: Distribution of types of homologous recombination deficiency, including germline and somatic BRCA and other variants which provide BRCAness to high‑grade epithelial ovarian carcinoma
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87-108
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363: 943-53
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA. et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950-5
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S. et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34-43
- Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S. et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2014; 15: 396-405
- Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E. et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol 2013; 14: 1020-6
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365: 2473-83
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G. et al. Aphase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365: 2484-96
- Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E. et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol 2015; 16: 928-36
- du Bois A, Floquet A, Kim JW, Rau J, del Campo JM, Friedlander M. et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014; 32: 3374-82
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379: 2495-505
- Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M. et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 2019; 381: 2403-15
- González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR. et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019; 381: 2391-402
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martin A, Sevelda P. et al. Phase III PAOLA-1/ENGOT-ov25: maintenance olaparib with bevacizumab in patients with newly diagnosed, advanced ovarian cancer treated with platinum-based chemotherapy and bevacizumab as standard of care. Ann Oncol 2017; 30(Suppl 5): v851-934
- Phase III PAOLA-1/ENGOT-ov25 trial: Olaparib Plus Bevacizumab as Maintenance Therapy in Patients with Newly Diagnosed, Advanced Ovarian Cancer Treated with Platinum-based Chemotherapy Plus Bevacizumab. https://oncologypro.esmo.org/Meeting-Resources/ESMO-2019-Congress/Phase-III-PAOLA-1-ENGOT-ov25-trial-Olaparib-plus-bevacizumab-bev-as-maintenance-therapy-in-patients-pts-with-newly-diagnosed-advanced-ovarian-cancer-OC-treated-with-platinum-based-chemotherapy-PCh-plus-bev Available from: [Last accessed on 2020 Jan 05]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M. et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009; 361: 123-34
- O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015; 60: 547-60
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S. et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition. Cancer Res 2006; 66: 8109-15
- Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008; 105: 17079-84
- Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244-50
- Matulonis UA, Penson RT, Domchek SM, Kaufman B, Shapira-Frommer R, Audeh MW. et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety. Ann Oncol 2016; 27: 1013-9
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274-84
- Hinde S, Epstein D, Cook A, Embleton A, Perren T, Sculpher M. et al. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health 2016; 19: 431-9
- du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N. et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2016; 17: 78-89
- Norquist BS, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner SI. et al. Mutations in homologous recombination genes and response to treatment in GOG 218: An NRG oncology study. Gynecol Oncol 2016; 141: 2
- Harter P, Johnson T, Berton-Rigaud D, Park SY, Friedlander M, Del Campo JM. et al. BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol Oncol 2016; 140: 443-9
- Rohit K, Gupta S, Rajappa SJ, Advani SH, Agarwal A, Aggarwal S. et al. A non-interventional, multicenter study to assess prevalence of BRCA1 and BRCA2 mutation among ovarian, primary peritoneal, and fallopian tube cancer patients in India. J Clin Oncol 2019; 37 (Suppl. 15) e13073
- Singh J, Thota N, Singh S, Padhi S, Mohan P, Deshwal S. et al. Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: Prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 2018; 170: 189-96
- Mannan AU, Singh J, Lakshmikeshava R, Thota N, Singh S, Sowmya TS. et al. Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: Implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet 2016; 61: 515-22
- Valarmathi MT, Sawhney M, Deo SS, Shukla NK, Das SN. Novel germline mutations in the BRCA1 and BRCA2 genes in Indian breast and breast-ovarian cancer families. Hum Mutat 2004; 23: 205
- Sharma S, Rajaram S, Sharma T, Goel N, Agarwal S, Banerjee BD. et al. Role of BRCA1 and BRCA2 gene mutations in epithelial ovarian cancer in Indian population: A pilot study. Int J Biochem Mol Biol 2014; 5: 1-0
- Matthew WK, Becker M, Neff C, Abkevich V, Jones JT, Hou X, Wang Y. et al. Use of homologous recombination deficiency (HRD) score to enrich for niraparib sensitive high grade ovarian tumors. J Clin Oncol 2015; 33 (Suppl. 15) 5532
- Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 2016; 22: 3764-73


 PDF
PDF  Views
Views  Share
Share

