Weekly paclitaxel in escalating doses in a patient with anthracycline-resistant, triple-negative, metastatic breast cancer with severe liver dysfunction
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(03): 170-172
DOI: DOI: 10.4103/0971-5851.103145
Abstract
Liver dysfunction in a patient with anthracycline-resistant breast cancer and liver metastases with poor performance status (PS) represents a serious situation. Taxanes are the drugs of choice, but once the transaminase enzyme levels are raised more than 10-times the upper limit of normal (>10 ULN), paclitaxel administration is contraindicated. We present the report of one such case who had a gratifying response to escalating doses of weekly paclitaxel thus suggesting that even patients with severe liver dysfunction can derive benefits from such a strategy. The patient, a 54-year-old lady with breast cancer metastatic to the liver and bones and previous receipt of anthracycline-based therapy, presented to us with a PS of 3. Her liver functions (LFT) were: serum bilirubin 2.2 mg% (0.3-1.1 mg%), aspartate aminotransferase 375 IU/L (0-25 IU/L), alanine aminotransferase 369 IU/L (0-35 IU/L) and alkaline phosphatase 363 IU/L (38-126 IU/L). She was started on weekly paclitaxel 20 mg/m 2 and zoledronate. After the first dose, the LFTs rose marginally but the skin lesions stabilized. Dose was subsequently escalated to 40 mg/m 2 . At the end of the 10th week, her PS improved to 1 and the disease showed a partial response. LFTs improved markedly. However, 5 days after the administration of the 13 th dose, the disease progressed and paclitaxel had to be discontinued. It is possible to derive maximum palliative benefit with escalating doses of weekly paclitaxel even in patients whose liver functions are deranged with transaminase levels (>10 ULN) and in whom conventional administration of paclitaxel is contraindicated.
Publication History
Article published online:
02 August 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Liver dysfunction in a patient with anthracycline-resistant breast cancer and liver metastases with poor performance status (PS) represents a serious situation. Taxanes are the drugs of choice, but once the transaminase enzyme levels are raised more than 10-times the upper limit of normal (>10 ULN), paclitaxel administration is contraindicated. We present the report of one such case who had a gratifying response to escalating doses of weekly paclitaxel thus suggesting that even patients with severe liver dysfunction can derive benefits from such a strategy. The patient, a 54-year-old lady with breast cancer metastatic to the liver and bones and previous receipt of anthracycline-based therapy, presented to us with a PS of 3. Her liver functions (LFT) were: serum bilirubin 2.2 mg% (0.3–1.1 mg%), aspartate aminotransferase 375 IU/L (0–25 IU/L), alanine aminotransferase 369 IU/L (0–35 IU/L) and alkaline phosphatase 363 IU/L (38–126 IU/L). She was started on weekly paclitaxel 20 mg/m2 and zoledronate. After the first dose, the LFTs rose marginally but the skin lesions stabilized. Dose was subsequently escalated to 40 mg/m2. At the end of the 10th week, her PS improved to 1 and the disease showed a partial response. LFTs improved markedly. However, 5 days after the administration of the 13th dose, the disease progressed and paclitaxel had to be discontinued. It is possible to derive maximum palliative benefit with escalating doses of weekly paclitaxel even in patients whose liver functions are deranged with transaminase levels (>10 ULN) and in whom conventional administration of paclitaxel is contraindicated.
INTRODUCTION
Liver dysfunction in a patient with triple-negative (estrogen, progesterone receptor-negative and her-2 neu negative) breast cancer due to liver metastases represents a potentially serious situation, especially when the patient is anthracycline resistant.
Breast cancer in the setting of liver of liver metastases and/ or hepatic dysfunction is a complex therapeutic dilemma. Not only is the prognosis poor, but toxicity related to therapy may be unpredictable due to altered drug clearance.
It is often accompanied by a sharp decline in performance status (PS) and a rapidly progressive disease course. Taxanes represent the next line of therapy.
Administration of paclitaxel is contraindicated once the liver functions are more than 10-times the upper limit of normal (>10 ULN).[1–6] Serious toxicity can be seen even with lesser degrees of liver dysfunction. Weekly paclitaxel has not been studied in the setting of severe liver dysfunction.[1–6] We present the report of one such case who had a gratifying response to escalating doses of weekly paclitaxel thus suggesting that patients with severe liver dysfunction can derive benefits from such a strategy.
CASE REPORT
The patient, a 54-year-old lady, was diagnosed with breast cancer 6 months back. She presented with an 8 cm lump in the right breast. A mobile axillary node 2 cm in size was also palpable. The metastatic work-up, including a chest radiograph (X-ray), ultrasound of the abdomen (USG) and a bone scan, was negative. She underwent modified radical mastectomy (MRM) along with axillary node dissection. The size of the tumor was 7 cm. Three of 13 axillary nodes contained tumor deposits. A diagnosis of pT3 N1 M0 infiltrating duct carcinoma, Grade 3 was made. Immunohistochemistry revealed the tumor to be triple negative (estrogen, progesterone receptor negative and her-2 neu negative).
She was then started on chemotherapy - FEC (fluorouracil 500 mg/m2, epirubicin 75 mg/m2 and cyclophosphamide 500 mg/m2). After three cycles, she complained of appearance of nodules at the incision site as well as backache. Physical examination revealed local recurrence with the presence of multiple skin nodules at the MRM incision site. The contrast-enhanced computerized tomography of the abdomen revealed multiple liver metastases, one large metastases in the right lobe 8 cm in dimension as well as five lesions 1–2 cm in size. A magnetic resonance imaging of the spine and pelvis revealed multiple skeletal metastases in the lumbar, sacral and left illiac regions.
She was then referred to our center. HerPS was 3. Her liver functions (LFTs) were deranged [Table 1], and were as follows: serum bilirubin (S. bil) 2.2 mg% (normal range – 0.3–1.1 mg%), aspartate aminotransferase (AST) 375 IU/L (normal range – 0–25 IU/L), alanine aminotransferase (ALT) 369 IU/L (normal range – 0–35 IU/L) and alkaline phosphatase (ALP) 363 IU/L (normal range – 38–126 IU/L). The prothrombin time was mildly deranged, 17 (test)/12 (control) s. There was no history of preceding liver disease. Blood tests for presence of hepatitis B surface antigen and serology for hepatitis C, A and E were noncontributory.
Table 1
Drug administration, dosage and liver function tests
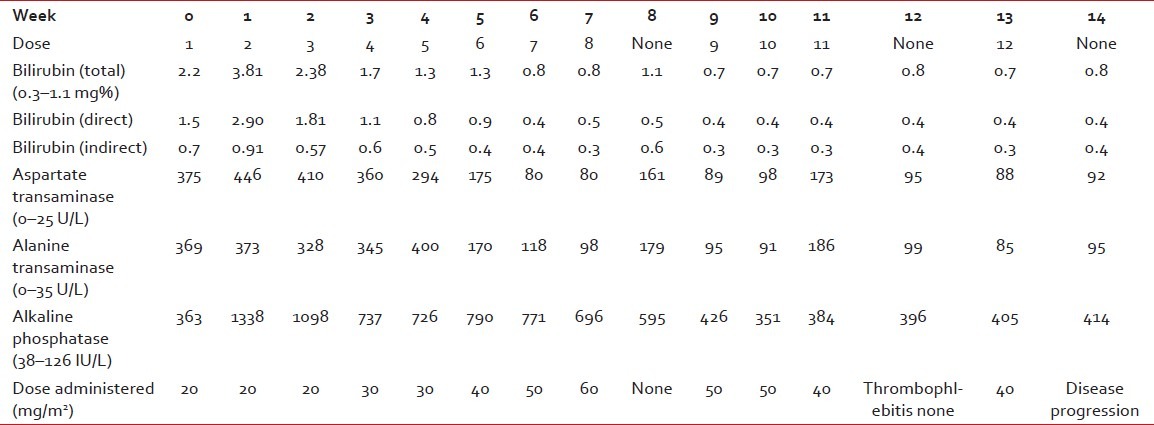
She was started on weekly paclitaxel 20 mg/m2 and zoledronate 4 mg every 4 weekly.
After the first dose of paclitaxel, LFTs showed mild deterioration. S.bil rose to 3.81 mg%, AST to 446 IU/L, ALT to 373 IU/L and ALP to 1338 IU/L. However, her PS improved to 2 and the skin lesions stopped progressing. The same dose was given for the next two cycles.
Her skin lesions showed signs of resolution and the LFTs improved. The dose was subsequently increased to 30 mg/m2. Steady improvements in the clinical status and LFTs were noted and dose increments of 10 mg/ m2 were made with each successive dose. The 60 mg/ m2 dose was associated with asymptomatic worsening of the LFTs. Hence, the dose was modified to 40 mg/ m2 [Table 1].
At the end of the 10th week, her PS had improved to 1. The skin lesions had disappeared. Radiological evaluation revealed a partial response. The 8-cm liver lesion had reduced to 3.5 cm in size. All the five lesions 1–2 cm in size initially had become less than I cm in dimension. The patient had no bone pains. There was no toxicity except grade 1 anemia – hemoglobin (Hb) 9.5 gm% (normal range 12–15 g/dL). Alopecia owing to receipt of the previous FEC administration persisted. The LFTs also improved. The regimen was extremely well tolerated.
The week 12 dose could not be administered owing to thrombophlebitis. However, 5 days after the administration of the Week 13 dose (40 mg/m2), the skin lesions reappeared.
Ultrasound of the liver revealed that the lesion had increased in size from 3.5 cm to 4.5 cm. In view of progressive disease, paclitaxel was discontinued and the patient was placed on single-agent carboplatin.
DISCUSSION
Data regarding safety of paclitaxel in patients with clinically significant liver impairment is sparse.
Studies of paclitaxel pharmacokinetics in patients with normal or mildly impaired liver function suggested a negative correlation between total bilirubin concentrations and paclitaxel elimination.[1–6]
Venook et al. described higher hematological toxicities in patients with liver dysfunction receiving paclitaxel every 3-weekly over 24 h and over 3 h, and suggested dose reductions as a means of overcoming this toxicity.[2] Wilson et al., in a combined Phase I/II study, studied the pharmacokinetics involved in a 96-h infusion of paclitaxel and found correlations between tumor involvement of the liver, aspartate aminotransferase and total bilirubin concentrations and reduced paclitaxel clearance in patients with advanced breast cancer. They suggested suitable dose adjustments: reduction of dose from 140 mg/m2 to 105 mg/m2 in such patients.[3] Joerger et al. found a direct relationship between liver impairment, paclitaxel elimination and susceptibility to neutropenia/thrombocytopenia, and suggested the need for further dose adaptations for patients with more severe liver impairment (approximately 60–80% cycles were complicated with Grade ¾ hematological toxicity in patients with higher grades of liver dysfunction). This study also included patients other than those with breast cancer.[4] Another such study recommended the dose of 70 mg/ m2 every 2 weeks.[5] Huizing and colleagues studied nine patients with advanced breast cancer receiving paclitaxel 250 mg/ m2 as a 3-h infusion with subsequent granulocyte–colony stimulating factor support. Two patients with liver function disturbances showed a tendency to higher paclitaxel and 6-alfa hydroxypaclitaxel AUC levels, with more pronounced neuropathy.[6] Because the recommended dose of the 3-weekly regimen in hepatic dysfunction is lower than the standard dose, we started with lower doses of the weekly regimen in our case than the standard dose of weekly paclitaxel and then escalated them according to patient tolerance and hepatic dysfunction, as revealed by the LFTs.
Overall, the incidence of serious adverse events, neutropenia and neutropenic fever are significantly lower in the weekly taxanes schedules than in the three-weekly schedules and, hence, represent a safer treatment option.[7]
CONCLUSION
Use of taxanes in the face of liver dysfunction and deteriorating PS is severely limited, and administration is contraindicated once the transminase levels are more than 10-times the ULN . Weekly paclitaxel has not been studied in the setting of severe liver dysfunction. We suggest that it is possible to provide significant palliation with minimal side-effects with escalating doses of weekly paclitaxel in such patients.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared


 PDF
PDF  Views
Views  Share
Share

