Acute Promyelocytic Leukemia Masquerading as Sero-negative Polyarthritis: Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(04): 386-389
DOI: DOI: 10.1055/s-0042-1743506
Abstract
Musculoskeletal manifestations as the sole presentation in acute leukemia is rare in adults. Acute promyelocytic leukemia (APML) is a subtype of acute myeloid leukemia (AML) with reported incidence of 10 to 15% of total AML cases. APML presenting as polyarticular arthritis has never been reported in the literature. We present an interesting case of 20-year-old male patient who manifested with polyarticular arthritis mainly of small joints as the initial presentation, followed by pancytopenia and eventually was diagnosed as a case of APML on bone marrow morphology and molecular analysis for PML-RARα transcript. He was successfully treated with all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO). Arthritis also resolved with complete remission of APML. Arthritis in a case with pancytopenia should promptly be evaluated prior to treatment with steroids and anti-metabolites. Arthritis can be a presenting manifestation of APML and responds to prompt management of leukemia as in other cases of leukemic arthritis.
Keywords
acute promyelocytic leukemia - arthritis - musculoskeletalConsent
Informed consent was taken from the patient.
Supplementary MaterialPublication History
Article published online:
01 September 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Musculoskeletal manifestations as the sole presentation in acute leukemia is rare in adults. Acute promyelocytic leukemia (APML) is a subtype of acute myeloid leukemia (AML) with reported incidence of 10 to 15% of total AML cases. APML presenting as polyarticular arthritis has never been reported in the literature. We present an interesting case of 20-year-old male patient who manifested with polyarticular arthritis mainly of small joints as the initial presentation, followed by pancytopenia and eventually was diagnosed as a case of APML on bone marrow morphology and molecular analysis for PML-RARα transcript. He was successfully treated with all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO). Arthritis also resolved with complete remission of APML. Arthritis in a case with pancytopenia should promptly be evaluated prior to treatment with steroids and anti-metabolites. Arthritis can be a presenting manifestation of APML and responds to prompt management of leukemia as in other cases of leukemic arthritis.
Keywords
acute promyelocytic leukemia - arthritis - musculoskeletalIntroduction
The incidence of acute promyelocytic leukemia (APML) is reported to be 0.32 cases per 100,000 population as per the SEER database.[1] APML is classified as acute myeloid leukemia (AML) with recurrent genetic abnormalities, as per the WHO (2008) and is a result of a balanced translocation t (15;17), which leads to the formation of fusion oncoprotein PML-RARA that leads to the arrest of normal myeloid differentiation at a promyelocyte stage. With the discovery of all-trans retinoic acid (ATRA) and arsenic trioxide as differentiating agents, the prognosis of APML has improved drastically with complete remission rates of > 90%.[2] [3] [4] However, such high cure rates are achievable only with timely diagnosis and vigorous management. The main presentation of APML includes fever, generalized weakness, bleeding, and thrombotic manifestations due to coagulopathy.[5] Though available literature has reported various cases of AML developing after rheumatoid or psoriatic arthritis,[6] [7] [8] arthritis as an initial presentation in APML has never been reported to the best of our knowledge.
Case Report
This study being a case report, a waiver off consent was applied to the institutional ethics committee (IEC) and the same was accepted by the IEC.
We report a case of 20-year-old male patient, laborer by occupation, who consulted various physicians with complains of fatigue and multiple joint pain, swelling, and stiffness of joints for 1 month. He was evaluated and treated with prescribed non-steroid anti-inflammatory drugs (NSAIDs) by the general physician; however, due to the persistence of his symptoms he was referred to immunology department of our hospital. General physical examination revealed pallor, no petechiae, ecchymosis, or lymphadenopathy. Musculoskeletal examination revealed right temporo-mandibular joint pain with jaw and mouth opening of one finger, right acromial tip enthesitis, dactylitis of left second toe ([Fig. 1A]), left extensor tenosynovitis ([Fig. 1B]), cervical spine local tenderness (C5-C8) present with restriction of movement in flexion (50°), extension (30°) and lateral rotation bilateral (30°). Systemic examination was with in normal limits.
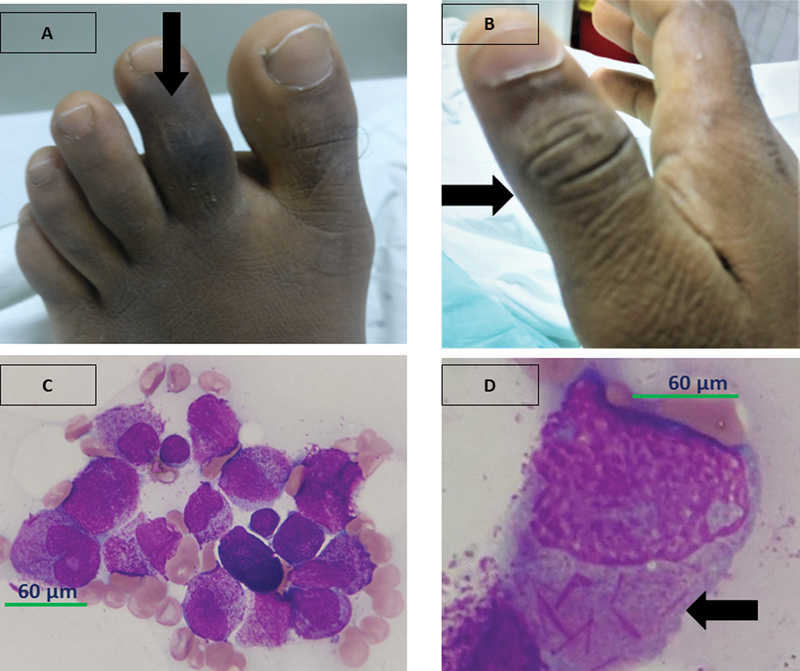
| Figure 1:(A and B) Arrow highlighting right thumb interphalangeal joint arthritis and dactylitis of the left second toe. (C and D) Normal hematopoietic elements are replaced by proliferation of more than 90%-abnormal promyelocytes having high N:C ratio, irregular nucleus, open chromatin, with 1–2 prominent nucleoli, moderate amount of pale basophilic cytoplasm, with many of them showing multiple Auer rods (“Faggot cells” arrow). Cytochemistry: These abnormal promyelocytes are strongly positive for MPO.
The laboratory investigations at first visit to the physician showed Hb-12.5 g/dL, total leucocyte count-4500/mm3, differential leucocyte count-neutrophil-62%, lymphocytes-38%, platelet count-120,000/µL, and peripheral blood smear-no abnormal cells. Baseline investigations at our center are documented in [Table 1]. Immunological work up showed elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), anti-nuclear antibody profile, rheumatoid factor, anti-citrullinated peptide antibody (ACPA), HLA-B27 were all negative. The X-ray of involved joints did not show any abnormality ([Supplementary figs. S1–3], available online only). Ultrasound of meta-tarso-phalangeal joint of the second left toe showed minimal effusion, which was not amenable for aspiration. Bone marrow and flow cytometry done at our center in view of pancytopenia were diagnostic of APML-macro-granular variant ([Fig. 1B], [1D]). RT-PCR for PML-RARα was positive for Bcr1 transcript ([Supplement file 1], available online only). Differentials of APML with leukemic arthritis versus reactive arthritis versus hemorrhagic arthritis were thought of after the reports were available. Hemorrhagic arthritis was ruled out due to normal coagulation parameters, no evidence of bleeding elsewhere and small joint involvement in this case contrary to typical large weight-bearing joint arthritis in hemorrhagic arthritis. The patient was labeled as APML, low risk (SANZ criteria),[3] and was started on ATRA at 45 mg/m2 per oral daily and ATO at 0.15 mg/kg/day IV infusion with close monitoring of coagulation parameters, electrocardiography (ECG), and serum electrolytes. In view of the limitation of activity due to arthritis and no relief with tramadol, the patient was started on low-dose prednisolone 10 mg daily but the symptoms and signs persisted, dose of steroids was increased to 15 mg and then 20 mg daily with some relief in symptoms. The patient tolerated ATRA and ATO protocol well with no adverse events and no differentiation syndrome. Day 28 marrow showed morphological remission. Musculoskeletal symptoms also gradually subsided and steroid was tapered and was discontinued by day 35 with complete resolution of musculoskeletal symptoms and signs. The patient continued consolidation with ATRA and ATO as per the APL0406 protocol.[4] Bone marrow morphology and RT-PCR for PML-RARα from marrow aspirate sample, post consolidation was negative. At 16 months of follow-up, the patient continues to be in remission without any recurrence of joint symptoms.
|
Parameters |
Investigations at our center at presentation |
Investigations post consolidation chemotherapy |
|---|---|---|
|
Hb (g/dL) |
9.1 |
11.3 |
|
TLC (/mm3) |
800 |
7200 |
|
DLC |
N5L20 |
N56L38E2M4 |
|
Platelet count (/µL) |
76,000 |
2,26,000 |
|
S. uric acid (mg/dL) |
4.2 |
NA |
|
S. calcium (mg/dL) |
9.1 |
NA |
|
S. phosphate (mg/dL) |
2.5 |
NA |
|
PT/INR |
15.4/1.34 |
NA |
|
APTT (seconds) |
26.7(Control-28.4) |
NA |
|
Fibrinogen (mg/dL) |
648 |
NA |
|
ESR (mm/hr) Westergren method |
140 |
18 |
|
Malarial parasite |
Negative |
NA |
|
Dengue serology |
Negative |
NA |
|
CRP (mg/dL) |
13.3 |
0.5 |
|
ANA |
Negative |
Negative |
|
ACPA |
Negative |
Negative |
|
RF |
Negative |
Negative |
|
HLA-B27 |
Negative |
Negative |
|
Bone marrow examination |
Hypercellular marrow with 90 |
References
- Guru Murthy GS, Szabo A, Michaelis L. et al. Improving outcomes of acute promyelocytic leukemia in the current era: analysis of the SEER database. J Natl Compr Canc Netw 2020; 18 (02) 169-175
- Lo-Coco F, Avvisati G, Vignetti M. et al; Gruppo Italiano Malattie Ematologiche dell'Adulto, German-Austrian Acute Myeloid Leukemia Study Group, Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369 (02) 111-121
- Sanz MA, Martín G, González M. et al; Programa de Estudio y Traitmiento de las Hemopatías Malignas. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood 2004; 103 (04) 1237-1243
- Platzbecker U, Avvisati G, Cicconi L. et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol 2017; 35 (06) 605-612
- Daver N, Kantarjian H, Marcucci G. et al. Clinical characteristics and outcomes in patients with acute promyelocytic leukaemia and hyperleucocytosis. Br J Haematol 2015; 168 (05) 646-653
- Buyukkurt N, Korur A, Boga C. Development of acute promyelocytic leukemia in a patient with gouty arthritis on long-term colchicine. Indian J Hematol Blood Transfus 2016; 32 (Suppl. 01) 80-81 DOI: 10.1007/s12288-015-0523-4.
- Ki MH, Ho LJ, Min LH. et al. A case of acute myeloid leukemia after adalimumab treatment in psoriatic arthritis. J Rheum Dis 2012; 19 (02) 91-94
- Tanaka K, Oshikawa G, Akiyama H. et al. Acute myeloid leukemia with t(3;21)(q26.2;q22) developing following low-dose methotrexate therapy for rheumatoid arthritis and expressing two AML1/MDS1/EVI1 fusion proteins: a case report. Oncol Lett 2017; 14 (01) 97-102
- Morais SA, du Preez HE, Akhtar MR, Cross S, Isenberg DA. Musculoskeletal complications of haematological disease. Rheumatology (Oxford) 2016; 55 (06) 968-981
- Evans TI, Nercessian BM, Sanders KM. Leukemic arthritis. Semin Arthritis Rheum 1994; 24 (01) 48-56
- Weinberger A, Schumacher HR, Schimmer BM, Myers AR, Brogadir SP. Arthritis in acute leukemia. Clinical and histopathological observations. Arch Intern Med 1981; 141 (09) 1183-1187
- Spilberg I, Meyer GJ. The arthritis of leukemia. Arthritis Rheum 1972; 15 (06) 630-635
- Needleman M. Childhood leukemia mimicking arthritis. J Am Board Fam Pract 1996; 9 (01) 56-60
- Silverstein MN, Kelly PJ. Leukemia with osteoarticular symptoms and signs. Ann Intern Med 1963; 59: 637-645
- Brix N, Rosthøj S, Herlin T. et al. Arthritis as presenting manifestation of acute lymphoblastic leukaemia in children. Arch Dis Child 2015; 100 (09) 821-825
- Thomas LB, Forkner Jr CE, Frei III E, Besse Jr BE, Stabenau JR. The skeletal lesions of acute leukemia. Cancer 1961; 14: 608-621
- Taillan B, Leyge JF, Fuzibet JG, Nectoux F, Ziegler G, Dujardin P. Knee arthritis revealing acute leukemia in a patient with rheumatoid arthritis. Clin Rheumatol 1991; 10 (01) 76-77
- Naithani R, Mahapatra M, Kumar R, Kumar A, Agrawal N. Arsenic trioxide induced acute flare-up of rheumatoid arthritis in a patient with APL. Ann Hematol 2007; 86 (02) 151-152
- Ayla Gokmen Akoz. Huseyin Engin & Nefise Oztoprak. Atypical presentation of retinoic acid syndrome that mimics septic arthritis in a patient with acute promyelocytic leukemia. Acta Oncol (Madr) 2007; 46 (08) 1193-1194
Address for correspondence
Rajesh Kashyap, MDDepartment of Haematology, Sanjay Gandhi Post Graduate Institute of Medical SciencesRaebareli Road, Lucknow 226014, Uttar PradeshIndiaEmail: rajkashyapmd@gmail.comPublication History
Article published online:
01 September 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:(A and B) Arrow highlighting right thumb interphalangeal joint arthritis and dactylitis of the left second toe. (C and D) Normal hematopoietic elements are replaced by proliferation of more than 90
References
- Guru Murthy GS, Szabo A, Michaelis L. et al. Improving outcomes of acute promyelocytic leukemia in the current era: analysis of the SEER database. J Natl Compr Canc Netw 2020; 18 (02) 169-175
- Lo-Coco F, Avvisati G, Vignetti M. et al; Gruppo Italiano Malattie Ematologiche dell'Adulto, German-Austrian Acute Myeloid Leukemia Study Group, Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369 (02) 111-121
- Sanz MA, Martín G, González M. et al; Programa de Estudio y Traitmiento de las Hemopatías Malignas. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood 2004; 103 (04) 1237-1243
- Platzbecker U, Avvisati G, Cicconi L. et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol 2017; 35 (06) 605-612
- Daver N, Kantarjian H, Marcucci G. et al. Clinical characteristics and outcomes in patients with acute promyelocytic leukaemia and hyperleucocytosis. Br J Haematol 2015; 168 (05) 646-653
- Buyukkurt N, Korur A, Boga C. Development of acute promyelocytic leukemia in a patient with gouty arthritis on long-term colchicine. Indian J Hematol Blood Transfus 2016; 32 (Suppl. 01) 80-81 DOI: 10.1007/s12288-015-0523-4.
- Ki MH, Ho LJ, Min LH. et al. A case of acute myeloid leukemia after adalimumab treatment in psoriatic arthritis. J Rheum Dis 2012; 19 (02) 91-94
- Tanaka K, Oshikawa G, Akiyama H. et al. Acute myeloid leukemia with t(3;21)(q26.2;q22) developing following low-dose methotrexate therapy for rheumatoid arthritis and expressing two AML1/MDS1/EVI1 fusion proteins: a case report. Oncol Lett 2017; 14 (01) 97-102
- Morais SA, du Preez HE, Akhtar MR, Cross S, Isenberg DA. Musculoskeletal complications of haematological disease. Rheumatology (Oxford) 2016; 55 (06) 968-981
- Evans TI, Nercessian BM, Sanders KM. Leukemic arthritis. Semin Arthritis Rheum 1994; 24 (01) 48-56
- Weinberger A, Schumacher HR, Schimmer BM, Myers AR, Brogadir SP. Arthritis in acute leukemia. Clinical and histopathological observations. Arch Intern Med 1981; 141 (09) 1183-1187
- Spilberg I, Meyer GJ. The arthritis of leukemia. Arthritis Rheum 1972; 15 (06) 630-635
- Needleman M. Childhood leukemia mimicking arthritis. J Am Board Fam Pract 1996; 9 (01) 56-60
- Silverstein MN, Kelly PJ. Leukemia with osteoarticular symptoms and signs. Ann Intern Med 1963; 59: 637-645
- Brix N, Rosthøj S, Herlin T. et al. Arthritis as presenting manifestation of acute lymphoblastic leukaemia in children. Arch Dis Child 2015; 100 (09) 821-825
- Thomas LB, Forkner Jr CE, Frei III E, Besse Jr BE, Stabenau JR. The skeletal lesions of acute leukemia. Cancer 1961; 14: 608-621
- Taillan B, Leyge JF, Fuzibet JG, Nectoux F, Ziegler G, Dujardin P. Knee arthritis revealing acute leukemia in a patient with rheumatoid arthritis. Clin Rheumatol 1991; 10 (01) 76-77
- Naithani R, Mahapatra M, Kumar R, Kumar A, Agrawal N. Arsenic trioxide induced acute flare-up of rheumatoid arthritis in a patient with APL. Ann Hematol 2007; 86 (02) 151-152
- Ayla Gokmen Akoz. Huseyin Engin & Nefise Oztoprak. Atypical presentation of retinoic acid syndrome that mimics septic arthritis in a patient with acute promyelocytic leukemia. Acta Oncol (Madr) 2007; 46 (08) 1193-1194


 PDF
PDF  Views
Views  Share
Share

