Clear cell carcinoma of ovary with squamous metaplasia: A unique histopathological observation
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(03): 177-179
DOI: DOI: 10.4103/0971-5851.92828
Abstract
Squamous metaplasia though is commonly associated with ovarian endometrioid neoplasm, mixed mullerian tumor and Brenner tumors; it has not been described in an ovarian clear cell carcinoma. Unusual presence of squamous islands, either in the form of morules or keratin plaques, may create diagnostic difficulty in a clear cell carcinoma of ovary, and thus the other common associations should be ruled out. Here, we describe a case of clear cell carcinoma of the ovary, with both gross and microscopically identifiable squamous metaplasia. To the best of our knowledge, this is the first report on this rare association and should be documented for wider awareness.
Publication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Squamous metaplasia though is commonly associated with ovarian endometrioid neoplasm, mixed mullerian tumor and Brenner tumors; it has not been described in an ovarian clear cell carcinoma. Unusual presence of squamous islands, either in the form of morules or keratin plaques, may create diagnostic difficulty in a clear cell carcinoma of ovary, and thus the other common associations should be ruled out. Here, we describe a case of clear cell carcinoma of the ovary, with both gross and microscopically identifiable squamous metaplasia. To the best of our knowledge, this is the first report on this rare association and should be documented for wider awareness.
INTRODUCTION
Clear cell carcinoma (CCC) comprises 2.4% of ovarian epithelial neoplasms.[1] A metaplastic squamous element (SM) has not been described in a CCC. SM on the other hand has been reported with adenofibromas, proliferating endometrioid tumors, 30-50% of endometrioid adenocarcinoma (EAC) and, rarely, with transitional cell carcinomas or malignant Brenner tumors.[2–4] Presence of unexpected SM in a CCC may thus create a great diagnostic difficulty.
CASE REPORT
A 50-year-old postmenopausal woman presented with abdominal distension and pain of 2 months duration. The Eastern Cooperative Oncology Group (ECOG) performance status was-1 and the other vitals were normal. Per abdomen examination revealed a firm irregular mass of 24 weeks size in the supra-pubic region. With per speculum examination a healthy cervix and per vagina examination, a mass was felt in the anterior fornix. Contrast-enhanced computed tomography (CAT) scan showed a pelvic mass measuring 30 × 15 × 15 cm in the central abdomen. Both ovaries were not separately identified from it. There was neither free fluid in the abdomen nor any pleural effusion or lymph node enlargement. Her cancer antigen (CA)-125 and serum carcinoembryonic antigen (CEA) levels were 83.8 U/ml and 6.15 ng/ml, respectively. She underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy and peritoneal wash cytology examination. Intraoperatively, there was a right ovarian cystic mass measuring 15 × 15 cm, which ruptured during removal and revealed clear serous fluid. The inner surface of the cyst showed large areas of pearly-white mucosa resembling the esophageal mucosa grossly ([Figure 1], arrow). The left ovary, uterus, and omentum were normal. The postoperative period was uneventful, and she was discharged on the sixth postoperative day.
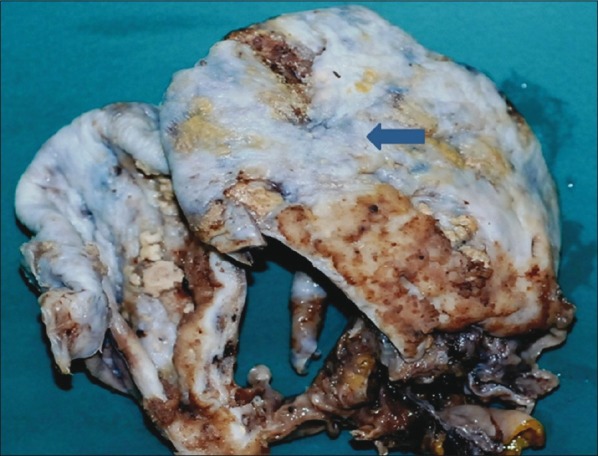
| Fig. 1 Gross photograph of the inner surface of the cystic ovarian tumor shows brownish-white papillary excrescences of the tumor along with extensive pearly-white glistening areas indicating metaplastic squamous epithelium (arrow)
Histopathological examination revealed features of a CCC showing solid sheets as well as papillae of large round to epithelioid cells, with nuclear hobnailing and moderate amount of clear cytoplasm and prominent cytoplasmic borders ([Figure 2a] [arrow] and and2b).2b). There were squamous morules, well identifiable squamous cells (black arrow), as well as extensive pools of keratin in the sections taken from the grossly identified pearly-white areas in the right ovary [Figure [Figure2b2b and andc].c]. Numerous multinucleated foreign body giant cells engulfing the keratinous material were identified ([Figure 2c], inset). Focally, both the squamous morules and keratin plaques showed preserved nuclei (arrow), nuclear ghosts and intercellular bridges [Figure 2d]. In addition, areas of stromal tumor infiltration and extensive stromal hyalinization were also evident. Peritoneal cytology examined was positive for malignancy. Extensive sampling was done to rule out any possible association with endometriosis or with a coexistent endometrioid carcinoma; however, neither was there any evidence of endometriosis nor areas of endometrioid-type adenocarcinoma. She was staged as carcinoma ovary, stage IC, and started on adjuvant chemotherapy using combination of Paclitaxel and carboplatin. Presently, she has completed three cycles of chemotherapy and has tolerated it well.
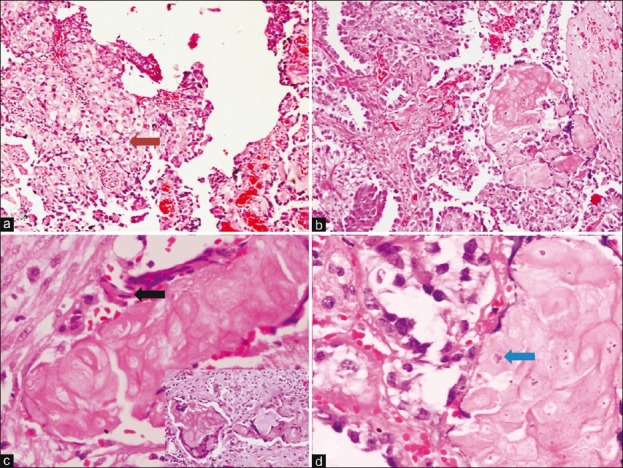
| Fig. 2 (a) Photomicrograph shows sheets of clear cells with cytoplasmic clearing [arrow] (H and E, ×100). Other areas show papillary pattern, where nuclear hobnailing. (b) Squamous morules are also seen (H and E, ×100); (c) there are flattened squamous cells [black arrow] as well as squamous cells with nuclear ghosts and intracellular bridging. Inset shows multinucleated giant cells with engulfed keratinous material (H and E, ×200; Inset: H and E, ×200). (d) Another area shows clear cells on the left and a squamous morule on the right with preserved nuclei [arrow] (H and E, ×200).
DISCUSSION
Metaplastic squamous islands as seen in association with the epithelial ovarian tumors, may be benign or show substantial cytological atypia, including foci of necrosis.[2,3] These islands may either be in the form of morules (SM with absence of keratinisation and intercellular bridges) or keratin plaques. They are commonly associated with EACs (30-50%). In the index case, focally identifiable squamous cell nuclei, intercellular bridges [Figure 2d], pools of extensive keratinisation, and gross appearance of the specimen clearly indicated the presence of a genuine SM.
EAC with secretary changes often mimics the hobnail pattern of CCCs and should be an important differential diagnosis.[1,5] The former shows columnar cells with supra or infra-nuclear vacuolization resembling a secretary endometrium. In contrast, the clear cells in CCC are polygonal; contain glycogen with abundant clear cytoplasm with true nuclear hobnailing. Rarely, an SM component of an EAC in which the squamous cells contain abundant glycogen can be confused with the cells of a CCC. Differentiation of this tumor from the other germ cell neoplasms showing clear cytoplasm, e.g., dysgerminoma, yolk sac tumor, or steroid cell tumors should also be considered morphologically. In this scenario, a proper clinical history, serum tumor markers as alpha-fetoprotein (AFP) or steroid hormone levels play a key role. Morphological absence of typical dysgerminoma areas, Schiller-Duval bodies, or steroid cells and immunonegativity for AFP helps to rule out these close differentials. Strong positivity for cytokeratin also rules out dysgerminoma, which had been observed in our case.[1]
Conventionally, to differentiate between CCC and EACs, the SM has been regarded as an indicator of the latter.[1] Our finding puts a question mark in the usefulness of this histo-morphological criterion to differentiate between these two neoplasms.
SM is also commonly observed in association with endometrioid adenofibromas; however, the association of adenofibromas and CCC of ovary is uncommon. In the index case, if both the CCC and SM components are arising from an adenofibroma, would remain a matter of debate. Van Bogaert observed multiple neoplasms in the form of a CCC in one ovary, an adenofibroma in the other ovary, a squamous cell carcinoma (SCC) in the uterine cervix, and uterine leiomyomas.[6] In this case also the adenofibroma may have been the forerunner of the CCC. Even after adequate sectioning, the absence of such adenofibromatous area in our case renders us unable to resolve this issue. Concurrent CCC and a SCC have also been reported by Jobo et al. in a setting of an underlying ovarian endometriosis.[7] However, such a lesion has not been described arising from the former. SM can often be seen in a transitional cell or Brenner tumor.[2] However, in the absence of a benign Brenner tumor component or typical transitional areas in our case, these possibilities could be excluded. SM of ovarian surface epithelium had also been seen in patients undergoing peritoneal dialysis;[8] which is however, is not a possibility in our case. Non-germ-cell origin primary SCC of the ovary can be seen in women of age 23-90 years.[1] These components are usually high-grade and show a varied architectural pattern, including papillary, cystic, insular, verruciform, or sarcomatoid.[9] These can arise in association with endometriosis or from a dermoid cyst.[1] As in our case, a prominent CCC component was identified, the possibility of a primary SCC did not come in differential diagnoses.
CONCLUSION
Metaplastic squamous cell islands can be seen in a CCC of the ovary. As many close differential diagnoses exist, clinical correlation and generous histological sampling are a must before arriving at this rare diagnosis.
ACKNOWLEDGMENT
We thank the Department of Pathology, All India Institute of Medical Sciences (AIIMS) for providing the facilities and resources. PD is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, for providing research fellowship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Gross photograph of the inner surface of the cystic ovarian tumor shows brownish-white papillary excrescences of the tumor along with extensive pearly-white glistening areas indicating metaplastic squamous epithelium (arrow)

| Fig. 2 (a) Photomicrograph shows sheets of clear cells with cytoplasmic clearing [arrow] (H and E, ×100). Other areas show papillary pattern, where nuclear hobnailing. (b) Squamous morules are also seen (H and E, ×100); (c) there are flattened squamous cells [black arrow] as well as squamous cells with nuclear ghosts and intracellular bridging. Inset shows multinucleated giant cells with engulfed keratinous material (H and E, ×200; Inset: H and E, ×200). (d) Another area shows clear cells on the left and a squamous morule on the right with preserved nuclei [arrow] (H and E, ×200).


 PDF
PDF  Views
Views  Share
Share

