Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(02): 117-122
DOI: DOI: 10.4103/0971-5851.158844
Abstract
Breast cancer, in India, is the second commonest cancer in females. Receptor status with ER/PR/Her 2 is now routinely done in patients with invasive carcinoma. The tumour suppressor gene, p53, is also present in most breast cancers. Proteins produced by a mutated p53 gene, accumulate in the nucleus of tumour cells and are detected by immunohistochemistry (IHC). We have undertaken this study with the aim to evaluate the ER, PR, HER-2 and p53 expressions in invasive breast carcinomas by IHC and to compare the HER-2 expression with various clinicopathological parameters. Materials and Methods: In this retrospective single institutional study from January 2001 to December 2010, 389 cases of histopathologically diagnosed infiltrating carcinoma of breast were evaluated taking into account various parameters like age, tumour size, grade, lymph node involvement, ER and PR. HER-2 and p53 was done in 352 cases. Results: The age range was 23-90 years with a mean of 50.7 years. Majority of tumours were T2 (79.6%) and Grade II (60.9%). Our data showed overall 47.6% ER, 48.8% PR, 29.6% HER-2 and 69.2% p53 positivity. There was no significant correlation between HER-2 and age, tumour size, lymph node status, ER, and PR. There was significant correlation between HER-2 and tumour grade (P = 0.031), p53 (P < 0.001). There was no inverse correlation between HER-2 and combined ER, PR status. Triple-negative breast cancers which constituted 22.7% of our cases did not reveal any correlation with various parameters. Conclusion: In our study, ER status was low, and incidence of p53 was high. These findings suggest that many of the tumours in Indian females may be of an aggressive type, and novel treatment approaches may be tried. We conclude that the assessment of all four markers is desirable.
Keywords
Breast cancer - estrogen receptor - human epidermal growth factor receptor-2 - immunohistochemistry - p53 - progesterone receptorPublication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Breast cancer, in India, is the second commonest cancer in females. Receptor status with ER/PR/Her 2 is now routinely done in patients with invasive carcinoma. The tumour suppressor gene, p53, is also present in most breast cancers. Proteins produced by a mutated p53 gene, accumulate in the nucleus of tumour cells and are detected by immunohistochemistry (IHC). We have undertaken this study with the aim to evaluate the ER, PR, HER-2 and p53 expressions in invasive breast carcinomas by IHC and to compare the HER-2 expression with various clinicopathological parameters.
Materials and Methods:
In this retrospective single institutional study from January 2001 to December 2010, 389 cases of histopathologically diagnosed infiltrating carcinoma of breast were evaluated taking into account various parameters like age, tumour size, grade, lymph node involvement, ER and PR. HER-2 and p53 was done in 352 cases.
Results:
The age range was 23-90 years with a mean of 50.7 years. Majority of tumours were T2 (79.6%) and Grade II (60.9%). Our data showed overall 47.6% ER, 48.8% PR, 29.6% HER-2 and 69.2% p53 positivity. There was no significant correlation between HER-2 and age, tumour size, lymph node status, ER, and PR. There was significant correlation between HER-2 and tumour grade (P = 0.031), p53 (P < 0.001). There was no inverse correlation between HER-2 and combined ER, PR status. Triple-negative breast cancers which constituted 22.7% of our cases did not reveal any correlation with various parameters.
Conclusion:
In our study, ER status was low, and incidence of p53 was high. These findings suggest that many of the tumours in Indian females may be of an aggressive type, and novel treatment approaches may be tried. We conclude that the assessment of all four markers is desirable.
INTRODUCTION
Breast cancer is the most common malignancy among women in the western world and in India second only to the carcinoma cervix.[1] Worldwide, breast carcinoma remains a major concern as a leading cause of death. Early and accurate diagnosis remains the most important determinant in the treatment and outcome.
It has long been recognized that some human breast cancers are hormone-dependent. Estrogen regulates the differentiation and proliferation of breast epithelial cells and interacts with the estrogen receptor (ER) in the nucleus. Prolonged exposure of estrogen is an important risk factor for cancer. Progesterone receptor (PR) expression in normal breast epithelium is regulated by ER.[2] Presence of ER, PR and human epidermal growth factor receptor-2 (HER-2) status in invasive breast carcinoma is now-a-days routinely estimated as these markers are considered to be important prognostic factors.[3] ER and PR status has been used for many years to determine a patient's suitability for endocrine therapy (tamoxifen). HER-2 encodes a membrane protein that is tyrosine phosphorylated after interaction with its ligands. HER-2 is helpful in predicting the response to trastuzumab.[4] Nearly one-third of breast cancers have mutations in the p53, a tumour suppressor gene. Tumours with mutant p53 are associated with high histological grade and clinical aggressiveness.[5] Literature suggests that over-expression of HER-2 and p53 may have an adverse effect in breast cancer.[6,7]
MATERIALS AND METHODS
In this retrospective study from January 2001 to December 2010 in a single institution from south India, there were 408 cases of invasive breast carcinomas. These 408 cases of breast carcinoma patients underwent primary surgery at the center during this period. They did not receive any prior chemotherapy, radiotherapy or hormone therapy. One hundred and seventy-one cases underwent surgery elsewhere and came for an opinion. We have not included these cases in the study as the fixation status of the sample was not known. In the present study, 389 cases of infiltrating duct cell carcinoma not otherwise specified (nos) diagnosed histopathologically were included. We excluded breast carcinoma cases from other health care centers, where paraffin blocks of were received only for review and immunohistochemical analysis, with unknown fixation status. Postchemotherapy patients, in situ carcinomas and patients with incomplete information, were also excluded. The in-house specimens (modified radical mastectomy, lumpectomy, excisional biopsy, trucut) were fixed by 10% formalin prior to the year 2006 and 10% neutral buffered formalin after that period. The histopathology reports were accessed from the computerized hospital information system. We have taken into account the age, sex. There were 13 male patients with invasive ductal carcinoma, type of specimen and modified Scarf Bloom Richardson grade.[8]
Estrogen receptor, PR, HER-2, and p53 immunostain was done with both positive and negative controls. Tests were repeated whenever required. Immunostain for ER, PR, HER-2/neu, p53 was done on three-micron paraffin sections on 3-amino propyl ethoxy silane coated slides, with known positive controls as per manufacturer's instruction. Immunohistochemistry (IHC) was done by polymer horse radish peroxidase IHC detection system. Primary antibody used was monoclonal mouse antihuman antibody (ER-clone 1D5 in a dilution of 1:200, PR-clone PR 88 in a dilution of 1:200, HER-2/neu [c-erbB-2] clone CB11 in a dilution of 1:100, p53 clone D07 in a dilution of 1:1000). All the markers were from BioGenex. Antibodies from DAKO Company, Glostrup, Denmark were used prior to the year 2006. Antigen retrieval was done by pressure cooking for 5-10 min in Tris ethylenediaminetetraacetic acid buffer, pH 9.0 since the year 2006. Prior to this antigen retrieval was done by microwave based technique.
The slides were stained with 3, 3’-diaminobenzidine tetra hydrochloride chromogen, counterstained with Hematoxylin and mounted.
Nuclear staining was assessed for ER, PR, p53 and membrane staining was assessed for HER-2.
The intensity and proportion of tumour cells stained for ER and PR-were assessed by quick score system.[9,10] The normal epithelial elements served as an internal control.
Human epidermal growth factor-2 expression was determined based on the membrane staining pattern and scored on a scale of 0-3+ as recommended by the American Society of Clinical Oncology (ASCO) guidelines.[11] Scores of 0 and 1+ were considered negative for HER-2 protein expression. Tumours with scores of two or greater as positive for HER-2 over-expression were scored as positive for statistical consideration. The expression status of p53 was assessed according to the estimated proportion of nuclear staining of tumour cells that were positively stained. Tumours with 10% or greater nuclear positivity were considered to be positive for p53 protein accumulation.[5] For each case, 500 cells were counted; 200 cells in trucut biopsies [Figure].
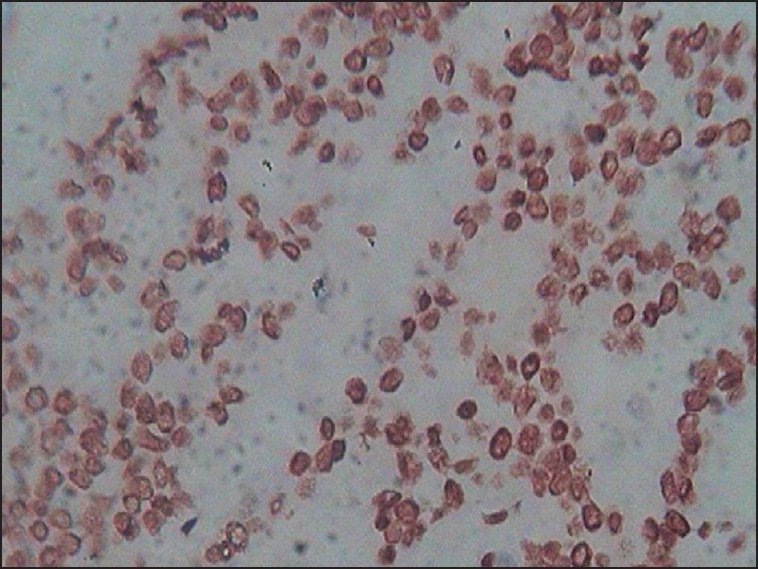
| Fig Infiltrating duct cell carcinoma of the breast (not otherwise specified) with nuclear staining for p53 (immunohistochemistry, ×40)
The data obtained was analyzed using the Statistical Package for the Social Sciences version 11.5 statistical program (SPSS Inc, Chicago). The institutional ethics board gave approval for the study.
RESULTS
The age range was wide (23-90 years) with a mean age of 50.7 ± 11.5 years. Maximum numbers of patients were over 50 years. Infiltrating duct cell carcinoma (nos) was the most common type (n = 389) followed by infiltrating lobular carcinoma. Other variants encountered include colloidal, papillary, metaplastic and adenoid cystic carcinoma.
Tumour size was available in 201 cases, out of which majority 160 (79.6%) belonged to T2 size (2-5 cm).
Using modified Scarf Bloom Richardson's grading majority 237 (60.9%) were Grade II followed by Grade III 137 (35.2%).[8]
Histopathological status of lymphnodes was available in 211 cases. Of these 138 (65.4%) showed metastatic deposits.
Our data showed overall 47.6% ER, 48.8% PR, 29.6% HER-2 and 69.2% p53 positivity [Table 1]. ER + PR + cases were 139 (35.7%), ER + PR-were 46 (11.8%), ER-PR + were 51 (13.1%) and ER-PR-was 152 (39.1%).
Table 1
Data on clinical, histological, pathological and receptor status in breast carcinomas — number (%)
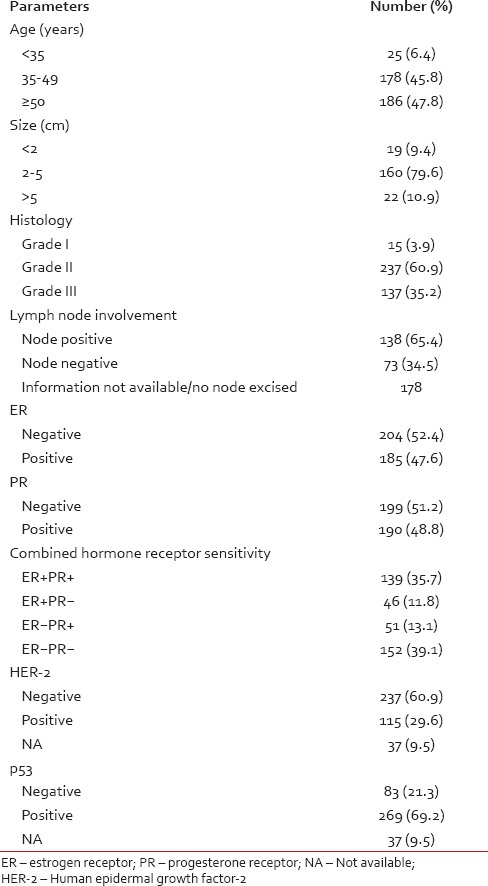
Pearson Chi-square test did not reveal any significant correlation between HER-2 and parameters like age, tumour size, lymph node status, ER, and PR. But, there a was significant correlation between HER-2 and tumour grade (P = 0.031), p53 (P < 0.001) and combined ER, PR status (P = 0.003).
Triple negative cancers, where all three of the receptors (ER, PR, and HER-2) are negative, constituted 22.7%. There was no significant correlation between above-mentioned parameters and triple negative cases in this study.
DISCUSSION
There is definite evidence that the incidence of breast cancer in India is on the rise.[1] Several risk factors for the development of the breast carcinoma have been established. The common denominator for most of the factors is prolonged estrogen stimulation operating on a genetically susceptible background.[2]
Presence of ER and PR in invasive carcinoma correlates positively with survival and is an important prognostic factor. The determination of ER, PR and HER-2 in breast cancer has become part of the standard workup.[3]
In this study, majority of patients (47.8%) were postmenopausal, over 50 years of age. Patients < 35 years constituted 6.4% of total. This finding also has been previously reported by other Indian studies.[3,12] These studies state that compared to their western counterparts, the mean age of Indian breast cancer patients are lower.[3] Age was determined as recorded in the clinical case records and pathology requisition. Patients’ age did not reveal any significant correlation with HER-2 in this study (P = 0.613). Similar findings have been reported by other Indian studies.[13,14]
Lymph nodal status was available in 211 patients. Metastatic lymphnodes were noted in 138 (65.4%) cases. No significant correlation was noted between HER-2 and lymph nodal status (P = 0.383). This finding is in contrast to that noted by Vaidyanathan et al.[13]
Tumour size was available in 201 cases. Majority of tumours, 160 (79.6%) belonged to T2 category size (2-5 cm). There was no significant correlation between HER-2 status and tumour size (P = 0.241). This finding is similar to that noted by Ambroise et al.[3] Primary surgery was done in 408 patients. These patients did not receive any prior chemo/radio/hormonal therapy. However, Vaidyanathan et al. and Kumar et al. reported a positive correlation.[13,14]
The proportion of stained cells, Allred score, quick score and H score are the most commonly used methods to assess the hormone receptor status.[9,10] We used the quick score system to determine the extent and intensity of receptor status. A cut-off point corresponding to 10% of the stained cells was used to identify hormone receptor-positive tumours in various studies emphasizing the significant association between the proportion of cells stained and the response to endocrine therapy.[3,12,14,15,16,17] A quick score above two (above 1% of weakly positive cells) has been reported to predict responsiveness to endocrine treatment.[15,16,17] In 2001, a consensus development panel of US National Institute of Health purposed that any nuclear positivity should consider as positive for ER status so that the patient can avail endocrine therapy.[16]
We noted an overall 47.6% of ER and 48.8% PR positivity. The prevalence of hormone receptor-positive breast cancer in Asian countries has been found to be lower than the western world. Western studies have reported 70-80% ER and 60-70% PR expression in the case of invasive ductal carcinoma, respectively.[3,15,17,18] However, studies from India have documented lower positivity for both the receptors. Desai et al. from India have documented the prevalence of 32.6% for ER-positive and 46.1% for PR-positive breast cancers.[19] A recent study from Mumbai also showed that hormone receptor expression in India is lower compared to the west.[20]
The study by Munjal et al. has also revealed a lower figure.[12] Dutta et al. have reported overall 24% ER and 30% PR positivity in their article. The explanations of this lower positivity as given by the authors include parameters like difference in techniques of evaluation, high tumour grade, and postmenopausal females.[21]
Ambroise et al. in their study from South India have showed that 59% ER positivity and 51% PR positivity respectively.[3] Similarly, Mudduwa, in a study from Srilanka documented a prevalence of 45.7% ER-positive and 48.3% PR-positive tumours.[9]
The percentage of tumours expressing PR but not ER-was 13.1% in our study. This is higher in contrast to other Indian studies by Shet et al. and Ambroise et al., who have reported 3.4% and 4.05% for this category.[3,20] However in another Indian study by Kumar et al. ER negative and PR-positive tumours were 13.3%.[14]
Since its initial discovery, HER-2 was used as a therapeutic target because of the efficacy of trastuzumab, a recombinant humanized monoclonal antibody that binds with high affinity to HER-2, in advanced breast cancer.[4] Over-expression of HER-2 occurs through either amplification of the gene or mRNA over-expression. One study from north India had shown HER-2/neu oncogene over-expression is higher (46.37%) among Indian patients in comparison to 25-30% shown in most western literature.[14,15]
In this study HER-2 positivity was 29.6%. Our findings are similar to that noted by Ambroise et al. (27.10%).[3] It did not exhibit any correlation with ER and PR individually. However, there was a significant correlation between combined ER, PR status and HER-2 (P = 0.003). This is in contrast to Kumar et al. findings where a significant correlation between has been described between ER, PR receptor status and HER-2 over-expression[14] Ambroise et al. also could not describe an inverse relationship between HER-2 and ER, PR expression similar to this study.[3] Vaidyanathan et al. have noted 43.2% HER-2 positivity by IHC. This is quite high compared to western literature. They also found a significant correlation between ErbB-2 status and lymphnode status, tumour size and ductal carcinoma.[13]
Indian studies show considerable variation regarding the frequency of HER-2 positivity. Vaidyanathan et al. noted 43.2% HER-2 positivity by IHC and 25.5% by genomic polymerase chain reaction.[13] Whereas James et al. have documented 29% HER-2/neu positivity by IHC.[22] A study from Central India has found 40.2% of tumours to be HER-2/neu positive.[12] Another study from Varanasi revealed it to be 46.3%[21] [Table 2].
Table 2
Receptor status in various study groups from India
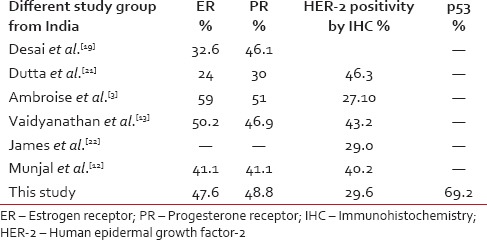
We have included those cases that were immunohistochemically 2+ or 3+ according to ASCO guidelines as HER-2 positive cases. The equivocal 2+ cases we have included under HER-2 positive cases for statistical purpose. We have not performed fluorescence in situ hybridization (FISH) in these cases.
We agree with Ambroise et al. regarding the fact that the frequency of HER-2 positivity may change if we take into account only the positive cases detected by FISH analysis. Again according to Vogel, though using the novel ASCO/College of American Pathologists criteria for assessing immunohistochemical results in HER-2 testing helps to reduce the IHC false positive rate of HER-2, in heterogeneous carcinomas, even FISH may not succeed in a correct evaluation of HER-2. However, FISH remains the gold standard for assessment of HER-2 status.[23]
Accordingly, in a recent article from south India the authors have opined that IHC HER-2 equivocal (2+) cases are a heterogeneous group and FISH is helpful in categorizing these tumours.[24]
Another marker that we have included in our study is p53, a tumour suppressor gene, which is located on the short arm of chromosome 17. P53 is involved in regulating cell proliferation, inducing apoptosis, and in promoting chromosomal stability. Disruption of these functions appears to play an important role in carcinogenesis. P53 over-expression has been observed in 20-50% of primary breast tumours.[5] Because proteins produced by a mutated p53 gene are not degraded as quickly as the wild-type protein, p53 proteins tend to accumulate in the nucleus of tumour cells, and can be detected by IHC.[5]
In view of the recent available literature, that the mutant p53 can be targeted; assessment of p53 has assumed significance.[25,26]
There is also evidence from the literature that, coexistence of over-expression of HER-2 and p53 protein accumulation is a strong prognostic marker in breast cancer.[25,26,27]
Lee et al. have noted 37.1% p53 over-expression in their study.[5] In our data, we noted 69.2% p53 positivity. We also noted significant positive correlation between HER-2 and p53 (P < 0.001).
Triple-negative breast cancers (TNBCs) have recently attracted a lot of attention, because of their poor prognosis. TNBCs is frequently shown to harbor mutations in Tp53, resulting in loss of the G1 checkpoint and reliance on checkpoint kinase-1 to arrest cells in response to DNA damage. We encountered 22.7% triple negative cases, but statistically there was no significant correlation between various parameters including p53 with TNBC.
Definitely, further studies are required in larger groups taking into account various clinical parameters along with molecular study and survival pattern analysis to substantiate these immunohistochemical findings. All these factors may point to the fact that in the sub continental women, there is relatively low-expression of ER and high-expression of HER-2 and p53 (in resected tumours). This may be attributed to the aggressive form of the disease and racial differences.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Aparna Bitla for her invaluable help and senior technicians Mrs. Ushanandini and Mr. Ramana for their help.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig Infiltrating duct cell carcinoma of the breast (not otherwise specified) with nuclear staining for p53 (immunohistochemistry, ×40)


 PDF
PDF  Views
Views  Share
Share

