Metastatic Lobular Carcinoma of the Male Breast Masquerading as a Pancreatic Head Mass, a Diagnostic Dilemma—Rare Case and Literature Review
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(01): 124-128
DOI: DOI: 10.1055/s-0042-1742639
Abstract
Male breast cancer comprises of 1%-of all and invasive lobular carcinomas (ILC) are even rarer in males. ILC are known to have unusual metastatic sites. We report a case of a 51-year-old male diagnosed with breast cancer, who presented with a recurrent chest wall nodule and icterus after 24 months of disease-free interval. On further investigations, he was found to have pancreatic head mass associated with conjugated hyperbilirubinemia suggestive of obstructive jaundice and a left parasternal soft tissue recurrence. A self-expandable metallic stent was inserted for recurrent cholangitis. Biopsy from the chest wall nodule was recurrence of ILC and pancreatic head mass was suspected to be either a second primary or an isolated pancreatic head metastasis of ILC on imaging. In either case surgical resection if operable and localized was planned. However, on staging laparoscopy, the patient was found to have mild ascites and multiple peritoneal nodules, which on biopsy proved to be metastases from ILC. Patient was treated with second-line hormonal therapy with luteinizing hormone-releasing hormone agonist and an aromatase inhibitor. ILC may present with unusual sites of metastasis leading to diagnostic dilemma. A high index of suspicion of metastases and appropriate biopsies can help one embark upon the most appropriate plan.
Keywords
invasive lobular carcinoma - male breast cancer - pancreatic metastasis of lobular carcinomaPublication History
Article published online:
15 February 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Male breast cancer comprises of 1%-of all and invasive lobular carcinomas (ILC) are even rarer in males. ILC are known to have unusual metastatic sites. We report a case of a 51-year-old male diagnosed with breast cancer, who presented with a recurrent chest wall nodule and icterus after 24 months of disease-free interval. On further investigations, he was found to have pancreatic head mass associated with conjugated hyperbilirubinemia suggestive of obstructive jaundice and a left parasternal soft tissue recurrence. A self-expandable metallic stent was inserted for recurrent cholangitis. Biopsy from the chest wall nodule was recurrence of ILC and pancreatic head mass was suspected to be either a second primary or an isolated pancreatic head metastasis of ILC on imaging. In either case surgical resection if operable and localized was planned. However, on staging laparoscopy, the patient was found to have mild ascites and multiple peritoneal nodules, which on biopsy proved to be metastases from ILC. Patient was treated with second-line hormonal therapy with luteinizing hormone-releasing hormone agonist and an aromatase inhibitor. ILC may present with unusual sites of metastasis leading to diagnostic dilemma. A high index of suspicion of metastases and appropriate biopsies can help one embark upon the most appropriate plan.
Keywords
invasive lobular carcinoma - male breast cancer - pancreatic metastasis of lobular carcinomaKey Message
Invasive lobular carcinoma has unusual sites of metastasis. A high index of suspicion and use of preoperative biopsies for accurate diagnosis in the metastatic setting help in appropriate management of such patients and avoids unnecessary morbid surgery.
Introduction
Male breast cancer (MBC) comprises 1%-of all. And infiltrating lobular carcinoma (ILC) comprises 1%-of all MBC as compared to 10 to 15%-of female breast cancers (FBC).[1] [2]
Sanchez et al[3] reported the first case of ILC of the male breast in a patient with Klinefelter syndrome. A larger percentage of MBC ILC present with metastatic disease, often due to low index of suspicion on the part of patient and clinician alike. Metastatic pattern of ILC differs from that of infiltrating breast carcinoma, no special type (IBC-NST). Unlike common metastatic sites like lung, liver, and bone, ILC tend to metastasize to unusual sites like gastrointestinal tract, peritoneum, retroperitoneum, and leptomeninges.[4] A large retrospective study analyzed radiological imaging of 116 patients of ILC to understand their pattern of intra-abdominal metastasis. Peritoneum, liver, bowel, ovary, retroperitoneum, ureter, and lymph nodes were the common sites of metastasis in descending order.[5] ILC are generally of lower grade, have low mitotic index, and are hormone receptor (HR) positive with good response to hormonal therapy. However, there is conflicting evidence with respect to their prognosis as compared to IBC-NST with some studies showing better and others showing a worse outcome.[6] Patients of ILC with abdominal metastases seem to have a shorter overall and disease-free survival.[2] We present a rare case of MBC with ILC presenting with obstructive jaundice due to pancreatic head metastasis.
Case History
A 51-year-old man was diagnosed with left-sided breast cancer. He underwent a radical mastectomy, which on histopathology showed ILC and two positive axillary lymph nodes ([Supplementary Fig. S1A], available online only); estrogen receptor/progesterone receptor (ER/PR) was positive (Allred score—7/8 and 5/8, respectively) and HER 2 was negative ([Supplementary Fig. S1B–E], available online only). He received standard anthracycline and paclitaxel-based adjuvant chemotherapy. He also received locoregional radiation to chest wall and supraclavicular fossa. Patient was on maintenance tamoxifen when he presented with jaundice and anorexia after a disease-free interval of 2 years. On clinical examination, he had icterus and a 2 × 2 cm fixed chest wall lesion at the second costochondral junction. Biochemistry investigations revealed conjugated hyperbilirubinemia (total bilirubin—28 mg/dL, conjugated bilirubin—11mg/dL, serum glutamic-oxaloacetic transaminase—256IU/L, serum glutamic-pyruvate transaminase—109IU/L, alkaline phosphatase—249mU/mL) suggestive of obstructive jaundice. Serum CA-19.9 was within normal limits (24.35 U/mL). Abdominal ultrasound showed central intrahepatic biliary tract dilatation with a dilatated common bile duct (CBD) of 13 mm with an abrupt cutoff at the lower end. Magnetic resonance cholangiopancreatography was suggestive of a lesion in the head of pancreas measuring 9 × 8 mm involving the intrapancreatic CBD with upstream dilatation of suprapancreatic CBD, common hepatic duct, and intrahepatic biliary tract ([Fig. 1A]). A staging positron emission tomography-contrast-enhanced computed tomography showed increased uptake in an ill-defined soft tissue mass involving intrapancreatic bile duct and adjacent pancreatic parenchyma ([Fig. 1B]) (SUVmax—7.77) and a left parasternal soft tissue mass (SUVmax—5.88) ([Fig. 1C]). There was no other uptake on positron emission tomography in any other organ. A self-expandable metallic biliary stent was placed for recurrent cholangitis. Biopsy of chest wall lesion was recurrent ILC ([Fig. 1D]) and ER/PR was positive ([Fig. 1G, H]), consistent with that of the primary breast tumor. Radiological impression was that of an isolated pancreatic head mass. Two differentials of solitary pancreatic metastasis of lobular carcinoma and a second primary pancreatic head carcinoma were kept in mind. However, isolated pancreatic head metastasis from BC is extremely rare. In case of BRCA1 and BRCA2 mutation carrier incidence ratio for second pancreatic cancer reported to be 2.55 and 2.13, respectively.[7] On germline mutation analysis with hotspot common mutation testing with focused sequencing the patient had no pathogenic BRCA1 or 2 germline mutation. Staging laparoscopy was planned to be followed by a pancreaticoduodenectomy if resectable and localized pancreatic lesion along with wide excision of chest wall recurrence. However, on laparoscopy, he was found to have multiple subcentimetric peritoneal nodules with mild ascites with peritoneal carcinomatosis index >10. Multiple peritoneal biopsies were taken and ascitic fluid was sent for cytology. A decision to defer definitive treatment was made awaiting final histopathology report of the intra-abdominal biopsies. The histopathology of peritoneal nodule ([Fig. 1E]) and cytopathology of ascitic fluid cytology and cell block ([Fig. 1F]) proved the intra-abdominal disease to be recurrence and pancreatic metastasis of lobular carcinoma rather than a second primary. Patient was deemed to have metastatic BC and started on next line of hormonal therapy, that is, luteinizing hormone-releasing hormone (LHRH) agonist and aromatase inhibitor. The patient further progressed after 3 months and was started on weekly paclitaxel. He finally succumbed to disease after 5 months.
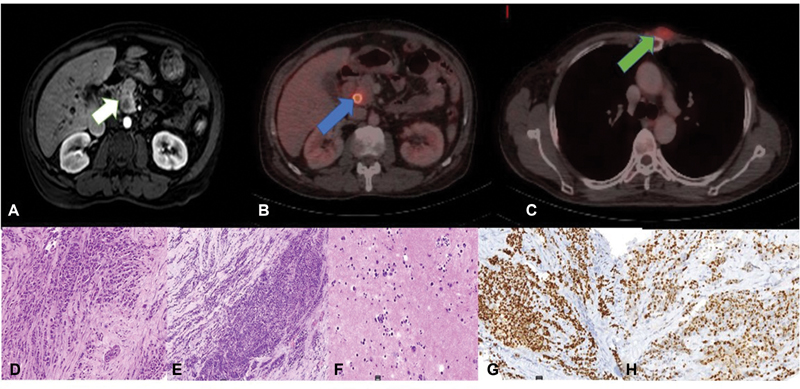
| Figure. 1 Imaging and histopathological features of recurrent lobular carcinoma: (A) Post-contrast magnetic resonance image shows a peripherally enhancing mass in the head of pancreas with moderate upstream intrahepatic biliary radical dilatation (white arrow). (B) Fused positron emission tomography computed tomography (PET/CT) shows fluorodeoxyglucose avid lesion at the head of pancreas (SUV max 7.77) with stent in situ (blue arrow). (C) Fused PET/CT shows the chest wall recurrence (SUV max 5.88) (green arrow). (D–F) Biopsy from chest wall recurrence, peritoneal nodule biopsy and peritoneal fluid cytology (hematoxylin and eosin, 20x). (G, H) Immunohistochemical markers of recurrent chest wall lesion diffuse staining for estrogen receptor and progesterone receptor.
Discussion
ILC is known to arise from terminal ductal and lobular epithelium in females and is often accompanied by lobular neoplasia. The occurrence of ILC is rare in males as they lack lobular development and hence little is known about the etiology of male ILC. The first few reports of ILC reported in male breast were in patients with Klinefelter syndrome.[8] A few common etiological factors are known to be those causing androgen/estrogen imbalance such as testicular dysgenesis as seen in Klinefelter syndrome, excessive alcohol intake, obesity, exogenous use of estrogen, chronic liver disease, etc. The breast parenchymal infiltration in ILC does not destroy the parenchyma or incite localized connective tissue response, hence fails to form palpable lump making early detection difficult.[9] In comparison with IBC-NST, ILC is significantly more likely to occur in older patients, to be larger in size, to be hormone receptor positive, to have lower S-phase fraction, to be diploid, and to be HER2, p53, and epidermal growth factor receptor negative.[10] One of the most consistent and characteristic is loss of cell-to-cell adhesion protein, E-cadherin. This feature near universally defines the discohesive histomorphological features of ILC.[2] The E-cadherin protein is coded by the CDH1 gene located on Chr16q21, a tumor suppressor gene maintaining cell-to-cell adhesion and tissue integrity.[11] The prognosis of ILC as compared to IBC-NOS seems to be contradictorily better or worse across literature.
Intra-abdominal metastases of ILC are known, the most common sites being peritoneum followed by liver, small bowel, and ovaries. ILC metastasis to gastrointestinal tract is like its spread in breast, i.e., it involves all the layers forming diffuse thickening instead of forming mass for e.g., in stomach, a linitis plastica like picture.[3] [10] A similar picture is seen in peritoneum and retroperitoneum where it forms tiny nodules instead of forming omental caking. Hence, these nodules are likely to be missed on imaging.[6] [12] Loss of E-cadherin is again responsible for the pattern of intra-abdominal spread of ILC. Moreover, the microenvironment of the ovary or peritoneum may provide growth and survival factors that favor ILC cells. Patients with intra-abdominal metastasis had a poorer overall and disease-free survival as compared to other sites of metastases.[13] Pancreatic metastasis due to other cancers is rare and from BC, it is even rarer.[9] Pancreatic metastasis of lobular carcinoma has been reported earlier either in synchronous or recurrent setting. Some of these patients were managed with synchronous surgery of primary BC and pancreatoduodenectomy.[10] Molino et al reported a patient who presented with obstructive jaundice and was found to have a breast lump on further investigations. She underwent pancreatoduodenectomy and mastectomy.[14] On final histopathological examination, the pancreatic mass showed metastasis from lobular carcinoma. In another case, the pancreatic head mass biopsy was suggestive of poorly differentiated adenocarcinoma but on final histopathology it turned out to be metastatic ILC.[15] Sun et al[6] also presented a case wherein the diagnosis of pancreatic metastasis was proven only after a definitive distal pancreaticosplenectomy when the tumor was subjected to a complete IHC panel. As such tissue diagnosis is not mandatory for radiologically resectable pancreatic cancer; hence, a preoperative biopsy of the pancreas is generally not performed.[16] In our case, there were two differentials—solitary metastasis (as no other lesions were seen on imaging) from ILC to pancreatic head or a second primary pancreatic tumor. In either case, a pancreatic head resection as a part of Whipple's surgery was deemed suitable for both scenarios; hence, a preoperative image-guided biopsy was not considered from the pancreatic head lesion. Our patient had no pathogenic germline mutation of BRCA1 or 2 which was discovered after subsequent testing, however the report was not available at the time of planning of treatment. A staging laparoscopy was planned to resolve the diagnostic ambiguity with an aim to carry out curative resection that on preoperative imaging was thought to be localized and resectable. Also, metastases from ILC to intra-abdominal organs may be radiologically indistinguishable from the primary site disease. However, based on our report and possibility of rare occurrences of pancreatic metastasis of lobular carcinoma, a pancreatic mass presenting either with breast lump or on follow-up in a patient previously treated for ILC may be evaluated for metastasis. A preoperative biopsy may also be suitable in such ambiguous situations. The knowledge of clinical history of ILC and its unusual metastatic presentations can direct appropriate IHC staining and help distinguish between metastasis and a new primary.[17] The role of tumor markers such as CA15.3, CA19.9, CEA is limited in such scenarios.[18] [Table 1] summarizes the previously published studies of pancreatic metastases in breast cancer, however none of them is reported in male breast cancer.
|
Study, year |
Number of cases |
Site of metastasis |
Management |
Survival (months) |
|---|---|---|---|---|
|
Azzarelli et al, 1982[21] |
1 |
Head of pancreas |
Pancreatoduodenectomy, RT |
72 |
|
Mehta et al, 1991[22] |
1 |
Head of pancreas |
Pancreatoduodenectomy |
27 |
|
Pappo et al, 1991[23] |
1 |
Head of pancreas and gall bladder |
Bypass and hormonal therapy |
18 |
|
Mountney et al, 1997[24] |
1 |
Head of pancreas |
Bypass and hormonal therapy |
24 |
|
Nomizu et al, 1999[25] |
1 |
Head of pancreas |
Pancreatoduodenectomy, chemotherapy, and hormonal therapy |
18 |
|
Le Borgne et al, 2000[26] |
1 |
Head of pancreas |
Pancreatoduodenectomy |
12 |
|
Moussa et al, 2004[27] |
1 |
Body of pancreas |
Total pancreatectomy |
7 |
|
Crippa et al, 2004[15] |
3 |
Head of pancreas |
Pancreatoduodenectomy |
28 |
|
Akashi et al, 2010[9] |
1 |
Head of pancreas |
Pancreatoduodenectomy |
28 |
|
Bonapasta et al, 2010[28] |
1 |
Head of pancreas |
Pancreatoduodenectomy |
36 |
|
Pan et al, 2012[29] |
1 |
Head of pancreas |
Chemotherapy and hormonal therapy |
21 |
|
Bednar et al, 2013[30] |
1 |
Head of pancreas |
Pancreatoduodenectomy |
48 |
|
Molino et al, 2014[14] |
1 |
Head of pancreas |
Pancreatoduodenectomy and hormonal therapy |
12 |

| Figure. 1 Imaging and histopathological features of recurrent lobular carcinoma: (A) Post-contrast magnetic resonance image shows a peripherally enhancing mass in the head of pancreas with moderate upstream intrahepatic biliary radical dilatation (white arrow). (B) Fused positron emission tomography computed tomography (PET/CT) shows fluorodeoxyglucose avid lesion at the head of pancreas (SUV max 7.77) with stent in situ (blue arrow). (C) Fused PET/CT shows the chest wall recurrence (SUV max 5.88) (green arrow). (D–F) Biopsy from chest wall recurrence, peritoneal nodule biopsy and peritoneal fluid cytology (hematoxylin and eosin, 20x). (G, H) Immunohistochemical markers of recurrent chest wall lesion diffuse staining for estrogen receptor and progesterone receptor.
References
- Senger JL, Adams SJ, Kanthan R. Invasive lobular carcinoma of the male breast – a systematic review with an illustrative case study. Breast Cancer (Dove Med Press) 2017; 9: 337-345
- Lehr HA, Folpe A, Yaziji H, Kommoss F, Gown AM. Cytokeratin 8 immunostaining pattern and E-cadherin expression distinguish lobular from ductal breast carcinoma. Am J Clin Pathol 2000; 114 (02) 190-196
- Sanchez AG, Villanueva AG, Redondo C. Lobular carcinoma of the breast in a patient with Klinefelter's syndrome. A case with bilateral, synchronous, histologically different breast tumors. Cancer 1986; 57 (06) 1181-1183
- DiPiro PJ, Tirumani SH, Cruz GP, Ramaiya NH, Lester SC, Shinagare AB. Lobular breast cancer: patterns of intraabdominal metastatic spread on imaging and prognostic significance. Abdom Radiol (NY) 2019; 44 (01) 362-369
- Winston CB, Hadar O, Teitcher JB. et al. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 2000; 175 (03) 795-800
- Sun X, Zuo K, Huang D, Yu B, Cheng Y, Yang W. Pancreatic metastasis from invasive pleomorphic lobular carcinoma of the breast: a rare case report. Diagn Pathol 2017; 12 (01) 52 DOI: 10.1186/s13000-017-0641-4.
- Iqbal J, Ragone A, Lubinski J. et al; Hereditary Breast Cancer Study Group. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012; 107 (12) 2005-2009
- Sousa B, Moser E, Cardoso F. An update on male breast cancer and future directions for research and treatment. Eur J Pharmacol 2013; 717 (1-3): 71-83
- Akashi Y, Saiura A, Kishi Y. et al. Outcome after surgical resection of isolated metastases to the pancreas. Hepatogastroenterology 2010; 57 (104) 1549-1552
- Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer 1984; 50 (01) 23-30
- Ciriello G, Gatza ML, Beck AH. et al; TCGA Research Network. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015; 163 (02) 506-519
- Yeatman TJ, Cantor AB, Smith TJ. et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg 1995; 222 (04) 549-559 , discussion 559–561
- Wernberg JA, Yap J, Murekeyisoni C, Mashtare T, Wilding GE, Kulkarni SA. Multiple primary tumors in men with breast cancer diagnoses: a SEER database review. J Surg Oncol 2009; 99 (01) 16-19
- Molino C, Mocerino C, Braucci A. et al; Breast Unit Cardarelli Hospital, Naples, Italy. Pancreatic solitary and synchronous metastasis from breast cancer: a case report and systematic review of controversies in diagnosis and treatment. World J Surg Oncol 2014; 12: 2 DOI: 10.1186/1477-7819-12-2.
- Crippa S, Bonardi C, Bovo G, Mussi C, Angelini C, Uggeri F. Pancreaticoduodenectomy for pancreatic metastases from breast carcinoma. JOP 2004; 5 (05) 377-383
- Larghi A, Rimbaş M, Rizzatti G. et al. Resectable pancreatic solid lesions: time to move from surgical diagnosis?. Endosc Ultrasound 2020; 9 (02) 76-82
- Reddy S, Edil BH, Cameron JL. et al. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol 2008; 15 (11) 3199-3206
- Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value?. Clin Chem 2006; 52 (03) 345-351
- Neifeld JP, Meyskens F, Tormey DC, Javadpour N. The role of orchiectomy in the management of advanced male breast cancer. Cancer 1976; 37 (02) 992-995
- Nance KV, Reddick RL. In situ and infiltrating lobular carcinoma of the male breast. Hum Pathol 1989; 20 (12) 1220-1222
- Azzarelli A, Clemente C, Quagliuolo V, Baticci F. A case of pancreatoduodenectomy as resolutive treatment for a solitary metastasis of breast cancer. Tumori 1982; 68 (04) 331-335
- Mehta SA, Jagannath P, Krishnamurthy SC, De Souza LJ. Isolated pancreatic metastasis from locally controlled breast cancer: a case report. Indian J Cancer 1991; 28 (01) 48-50
- Pappo I, Feigin E, Uziely B, Amir G. Biliary and pancreatic metastases of breast carcinoma: is surgical palliation indicated?. J Surg Oncol 1991; 46 (03) 211-214
- Mountney J, Maury AC, Jackson AM, Coleman RE, Johnson AG. Pancreatic metastases from breast cancer: an unusual cause of biliary obstruction. Eur J Surg Oncol 1997; 23 (06) 574-576
- Nomizu T, Katagata N, Matsuoka T. et al. A case of breast cancer metastatic to the head of the pancreas. Breast Cancer 1999; 6 (02) 131-134
- Le Borgne J, Partensky C, Glemain P, Dupas B, de Kerviller B. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology 2000; 47 (32) 540-544
- Moussa A, Mitry E, Hammel P. et al. Pancreatic metastases: a multicentric study of 22 patients. Gastroenterol Clin Biol 2004; 28 (10 Pt 1): 872-876
- Bonapasta SA, Gregori M, Lanza R. et al. Metastasis to the pancreas from breast cancer: difficulties in diagnosis and controversies in treatment. Breast Care (Basel) 2010; 5 (03) 170-173
- Pan B, Lee Y, Rodriguez T, Lee J, Saif MW. Secondary tumors of the pancreas: a case series. Anticancer Res 2012; 32 (04) 1449-1452
- Bednar F, Scheiman JM, McKenna BJ, Simeone DM. Breast cancer metastases to the pancreas. J Gastrointest Surg 2013; 17 (10) 1826-1831


 PDF
PDF  Views
Views  Share
Share

