Monitoring Measurable/Minimal Residual Disease in Acute Myeloid Leukemia: Multiparametric Flow Cytometry-Based Approach
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(06): 554-565
DOI: DOI: 10.1055/s-0043-1772203
Abstract
Measurable/minimal residual disease (MRD) status is the most relevant predictor of clinical outcome in hematolymphoid neoplasms, including acute myeloid leukemia (AML). In contrast to acute lymphoblastic leukemia, multiple myeloma, or chronic lymphocytic leukemia, etc., AML is a widely heterogeneous neoplasm with poor clinical outcomes. Multicolor flow cytometry (MFC) is a powerful technology with high sensitivity, rapid results, cost-effectiveness, and easy availability. It is routinely used for diagnosing and MRD monitoring in many hematological neoplasms. However, MFC-based MRD monitoring in AML is complex and challenging. It requires a refined approach, a wide panel of markers, and adequate training and experience. This review focuses on the panel design, processing details, template design, analysis approach, and recent updates in MFC-based MRD monitoring in AML. It further describes the normal distribution and maturation patterns of various sublineages among hematological progenitors and their utility in studying AML MRD.
Keywords
flow cytometry - minimal residual disease - acute myeloid leukemia
Supplementary MaterialPublication History
Article published online:
27 November 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Measurable/minimal residual disease (MRD) status is the most relevant predictor of clinical outcome in hematolymphoid neoplasms, including acute myeloid leukemia (AML). In contrast to acute lymphoblastic leukemia, multiple myeloma, or chronic lymphocytic leukemia, etc., AML is a widely heterogeneous neoplasm with poor clinical outcomes. Multicolor flow cytometry (MFC) is a powerful technology with high sensitivity, rapid results, cost-effectiveness, and easy availability. It is routinely used for diagnosing and MRD monitoring in many hematological neoplasms. However, MFC-based MRD monitoring in AML is complex and challenging. It requires a refined approach, a wide panel of markers, and adequate training and experience. This review focuses on the panel design, processing details, template design, analysis approach, and recent updates in MFC-based MRD monitoring in AML. It further describes the normal distribution and maturation patterns of various sublineages among hematological progenitors and their utility in studying AML MRD.
Keywords
flow cytometry - minimal residual disease - acute myeloid leukemiaIntroduction
Acute myeloid leukemia (AML) is a group of genetically heterogeneous disorders with unpredictable clinical outcomes. The diagnosis of AML is a complex process that requires the evaluation of multiple factors, including morphology, immunophenotype, and underlying genetics. AML largely affects older adults and has a poor prognosis, with only 35 to 40%-of patients younger than 60 years and 5 to 15%-of patients older than 60 years achieving long-term remissions.[1] [2] [3] [4]
Intensive chemotherapy has been the standard treatment for AML for many years, sometimes followed by allogeneic stem cell transplantation. The basis of improved outcomes in modern cancer therapy is risk-adopted therapeutic protocols where treatment intensity is modified based on the favorable risk versus high-risk disease. Genetic abnormalities form the basis for pretreatment prognostication in AML; however, they do not apply to some patients.[5] A robust factor for predicting disease-free survival (DFS) is an initial response to therapy, that is, complete remission (CR).[6] [7] [8] [9] [10] Morphologic CR, that is, less than 5%- blasts on cytomorphology in bone marrow (BM) aspirate, has been used as the clinical endpoint for evaluating chemotherapy efficacy. The cytomorphologic evaluation of CR has limitations, such as imprecision in quantifying myeloblasts using light microscopy counting up to 500 nucleated BM cells in a regenerating marrow and intra-/interobserver variability in identifying myeloblasts.[11] [12] In the last few years, it has been proved that the traditional method of evaluating CR through monitoring the percentage of blasts in the BM and peripheral blood using microscopic examination lacks the sensitivity to detect the leukemic blasts present at low levels.[11] [12] [13] Such low-level residual disease can be detected by only sensitive ancillary techniques such as flow cytometry or molecular methods (quantitative polymerase chain reaction [qPCR] and next-generation sequencing [NGS]) and is known as minimal/measurable residual disease (MRD).[14] [15] [16] Several studies suggest that identifying residual disease at levels far below the conventional 5%-blast threshold based on morphological analysis is a crucial tool in refining the approach to risk classification in leukemia.[17] [18] [19] [20] [21] [22] MRD refers to the detection of leukemia cells at levels as low as 1 in 10,000 to 1 in 1,000,000 white blood cells (WBCs), which is significantly lower than the 1 in 20 thresholds in morphology-based diagnosis.[23] [24] [25] Studies have shown that traditional morphologic CR may not effectively monitor initial therapeutic response in AML.[11] [12] [13] [26] [27] Several studies have demonstrated that MRD is a powerful predictor of DFS in AML.[14] [15] [16] It provides valuable information on disease response to the initial course of intensive therapy and helps predict outcomes in a given patient. Thus, it allows reliable monitoring of treatment effectiveness, identifying patients at high risk of relapse, and making informed decisions about the need for additional therapeutic intervention wherever possible.[17] [22] [25] [28] [29]
Over the past 20 years, there have been advancements in the methods for detecting AML MRD, such as multiparametric flow cytometry (MFC), qPCR, and NGS-based MRD methods.[23] [25] [29] [30] [31] [32] [33] [34] [35] [36] MFC is considered one of the most sensitive and specific methods for MRD detection in AML. This technology can detect even small numbers of residual leukemic cells among thousands of other hematopoietic cells, including normal myeloid progenitors, based on differences in cell surface markers. Major advantages of MFC-MRD over other techniques include easy availability, wider applicability (>90%-of AML), cost-effectiveness, and rapid turnaround time (TAT).[30] [35] [37] [38] Although theoretically, molecular methods can have higher sensitivity, due to limited applicability and the expensive nature of technology, practically, MFC-MRD has higher MRD detection sensitivity in most cases except in AML where MRD is performed by qPCR for NPM1 mutations.[39] [40] The sensitivity of MFC-MRD can be reached beyond 0.01%-in AML cases with definitive immunophenotypic aberrancies and in identifying cells with immunophenotype of leukemic stem cells (LSC). MFC-MRD not only can track the original clone of residual disease but also can easily identify new clones based on different-from-normal (DfN) approaches, which can be missed using molecular techniques based on targeted NGS or qPCR.[36] [39] Another valuable advantage of MFC-MRD is that it allows easy assessment of hemodilution and, thus, the quality of marrow being assessed for MRD detection. However, a major limitation of this method is that it is observer dependent and needs expertise with adequate experience and a standardized approach.[30] Another limitation of MFC-MRD is that it may not detect mature differentiating myeloid cells with genomic aberrancies, such as BCR::ABL1 in the CML-chronic phase or mature myeloid cells or monocytes with NPM1 mutations or PML::RARA in cases of acute promyelocytic leukemia.[36] [40] Recent studies have emphasized that incorporating AML MRD by using both MFC- and NGS-based MRD provides better prediction of clinical outcomes in AML.[3] [24] [29] [32] [36] [41] [42] This review is focused on an approach to develop antibody panels, standardization, template designing, and data analysis for MFC-MRD in AML.
Discussion
Designing of Antibody Panel
Selection of Markers
AML MRD assessment is a relatively complex and challenging assay and primarily based on two universal approaches, that is, identification of leukemia-associated immunophenotype (LAIP) and DfN antigen expression.[25] [29] [30] Unlike B-ALL MRD where residual leukemic cells primarily needs to be distinguished from normal B-cell precursors, residual disease in AML needs to be distinguished from a group of common myeloid progenitor cells differentiating toward granulocytic, monocytic, erythroid, dendritic cell, basophil, and mast cell precursor cells.[18] [20] [43] Hence, in addition to backbone markers as recommended by the European LeukemiaNet (ELN) working group for AML MRD (such as CD45, CD34, CD38, CD117, and HLADR), the panel needs markers that can identify myeloid progenitor cells differentiating toward different hematopoietic cells.[20] [25] [29] [38] Thus, antibody panel for AML MRD should also include CD13, CD15, and CD33 for granulocytic precursors; CD64, CD33, CD14, and CD36 for monocytic precursors; CD36 and CD71 for erythroid precursors; and HLADR, CD123, and CD203c for plasmacytoid dendritic cell, basophil, and mast cell precursors ([Supp. Fig. S1] and [Supp. Table 1], available in the online vesion). [Figs. 1] and [2] show the normal maturation stages of hematopoietic progenitors and their differentiation toward various sublineages with antigen expression patterns. The abnormal changes in the antigen intensities and asynchronous relation of these markers allow identification of aberrancies based on the DfN approach.[27] [35] [44] It should also include lymphoid markers commonly expression on leukemic cells for the detection of LAIP such as CD7, CD19, CD56, etc. Other rarely expressed lymphoid markers can also be included in the panel to further increase the applicability, such as CD2, CD4, CD5, CD11b, CD25, etc. A combination of CD34 and CD38 (CD34+ and CD38 +/− ) also allows for evaluation of the earliest hematopoietic precursors, commonly referred to as hematopoietic stem cells. Overexpression of antigens such as CD33 and CD123 and aberrant expression of CD7 and CD56 can help identify abnormal hematopoietic stem cells, also referred to as LSC.[45] [46] [47] [48] [49] Additional markers can be added to identify more aberrancies on committed progenitor cells and stem cells, including CD45RA, CD52, CD54, CD96, CD97, CD366 (Tim-3), CD371 (CLL-1), etc.[45] [46] [47] [48] [49]
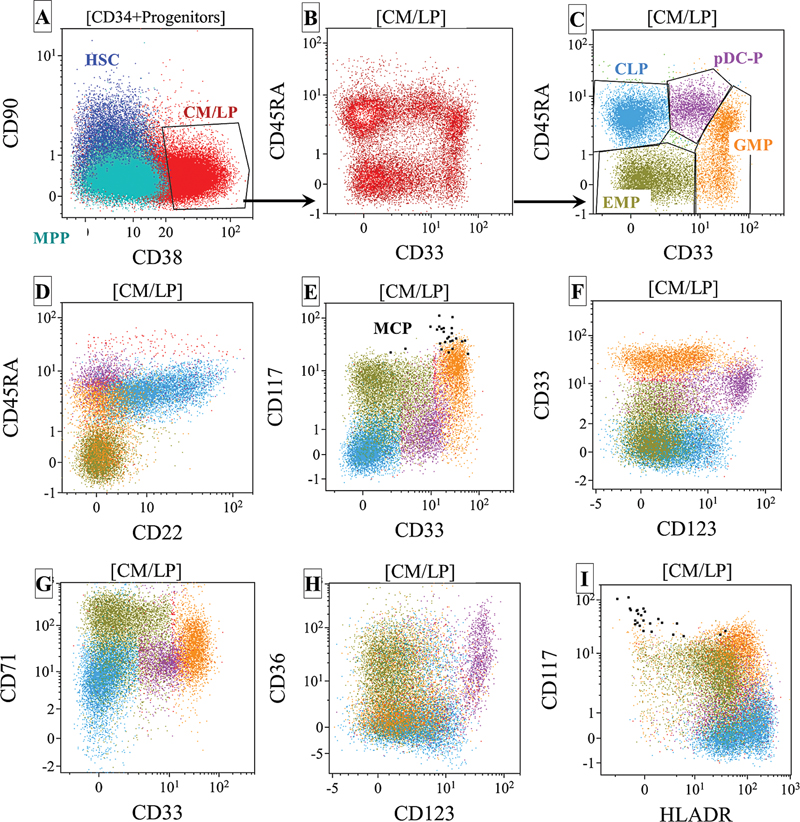
| Fig 1 : (A–I) Normal maturation stages of CD34+ hematopoietic progenitors and their differentiation toward various sublineages with antigen expression patterns. CLP, common lymphoid progenitors; CM/LP, common myeloid and lymphoid progenitors; CMP, common myeloid progenitors; GMP, granulocyte–macrophage (monocyte) progenitors; HSC, hematopoietic stem cells; MCP, Mast cell precursors; MPP, multipotent progenitors; MEP, megakaryocyte erythrocyte progenitors; pDC-P, plasmacytoid dendritic cell precursors.

| Figure 2:Normal maturation patterns of CD34+ hematopoietic progenitors with their differentiation toward various sublineages using commonly used antigen expression. Each dot plot shows the differentiation of hematopoietic stem cells (dark blue dots) toward the respective committed precursors using a combination of different markers. The maturation sequences are shown with black arrows. Red dots indicate granulocytic differentiation, cyan blue dots indicate monocytic differentiation, olive green dots indicate erythroid differentiation, light blue dots indicate B-cell precursors, pink dots indicate plasmacytoid dendritic cell precursors, and brown dots indicate basophil precursors. The gray color dots indicate the background mature mononuclear cells from a commonly used CD45 versus SSC progenitor gate (shown in [Fig. 1]). Abbreviations: Ba, basophils; BCP, B-cell precursors; CMP, common myeloid progenitors; HSC, hematopoietic stem cells; MPP, multipotent progenitors; PMy, precursor myeloid cells; PMo, precursor monocytes; PNB; precursor normoblasts; pDC, plasmacytoid dendritic cell.
Antibody Panel Designing
For any flow cytometry assay, panel designing is mainly based on the configuration of flow cytometer, allowing a maximum number of colors for simultaneous analysis. Recently the ELN working group for AML MRD has recommended a minimum eight-color flow cytometry requirement for AML MRD assessment.[29] However, the utilization of flow cytometers with more colors such as 10, 12, 13, or 16 colors, and so on, can improve the assay further. Flow cytometers with more colors allow simultaneous evaluation of more markers; hence, the detection of more abnormalities that can increase the assay's accuracy. The antibody panel of AML MRD requires a backbone of at least five common markers, that is, CD45, CD34, CD38, CD117, and HLADR.[38] A combination of these markers allows identification of progenitor cells and tracking of progenitor cells of interest in each tube. Other markers are required to identify progenitors differentiating toward various myeloid sublineages, identify deviation of antigen expression from normal, and detect LAIPs.[38] A common approach is to include those markers together, which can provide a sequence of maturation patterns of myeloid progenitor cells into sublineages. [Table 1] has shown a representative antibody combination that may be useful for AML MRD assessment on flow cytometers with various configurations. A recently published multicenter study suggested that adding a customized antibody combination based on the diagnostic immunophenotype can improve MRD results further.
Sample Preparation, Processing, and Acquisition
The ELN working group for AML MRD has also provided recommendations on the technical aspects of AML MRD in detail.[50] Briefly, BM aspirate samples are ideal for AML MRD assessment although studies evaluating MRD in peripheral blood have been published. Peripheral blood usually has one log lower MRD levels than BM samples and hence, there is a risk of false-negative results in peripheral blood MRD assessment. A common concern in quality BM sample for MRD reporting is hemodilution, which also may lead to false-negative results. Hence, the first-pull (0.5–1 mL) BM aspirate is highly recommended for MRD monitoring. Samples should be collected in ethylenediaminetetraacetic acid (EDTA) or heparin anticoagulants, transported at 8 to 20°C and processed within 48 to 72 hours of collection. A bulk-lysis-stain method as described by the EuroFlow Consortium is a preferred method of processing for MRD assay although the stain-lyse-wash method is also used in a few centers.[38] [51] An acquisition of a minimum of 500,000 CD45+ events per tube has been recommended; however, the target of sample acquisition should be collection of the highest possible number of relevant events. It is always encouraged to acquire at least 1 to 2 million cells to increase the sensitivity of MRD assay and a minimum of 100 events of abnormal blasts to provide confidence in detecting MRD.[18] [29] [30] [35]
Approach to MRD Analysis
An MFC-MRD analysis in AML is based on the integration of two approaches: (1) the LAIP approach and (2) the DfN approach.[29] [30] Commonly available LAIPs can be divided into three groups. Group 1 focuses on the aberrant expression of lymphoid markers (e.g., CD2, CD7, CD11b, CD19, CD56, etc.), group 2 on the absence or overexpression of myelomonocytic markers (e.g., CD13, CD15, CD33, CD36, CD64, and CD71), and group 3 on aberrancies that include over-/underexpression of markers such as CD34, CD38, CD117, CD123, HLADR, etc.[20] [38] [52] These aberrancies are identified in diagnostic samples; hence, it is easy to follow in the MRD assessment. Sometimes, the residual leukemic blasts may show deviation in LAIP compared to diagnostic samples due to an immunophenotypic shift during therapy or predominantly show the immunophenotype expressed on a small subset of blasts at diagnosis due to heterogeneity in antigen expression.[38] [53] [54] [55] [56] Hence, evaluating multiple LAIPs at diagnosis is recommended to avoid false-negative results due to the shift or absence of one LAIP. Consideration of at least two LAIPs to confirm MRD has been recommended.[30] [38] [57] A limitation to MRD assessment based on the LAIP-based approach is the need for a diagnostic immunophenotype, which may not be available sometimes, especially in stand-alone laboratories that receive referred samples. CD34+ progenitors from regenerating BM usually show weak expression of CD7 and sometimes CD56 and can lead to false-positive results.[4] Further, in a few cases, the LAIP approach may cause false-negative results if a new leukemic clone emerges during therapy or a chemoresistant subclone (less evident at diagnosis) persists. The DfN approach is very useful for MRD detection in such a scenario.
The DfN approach focuses on the difference in sequential expression of various antigens from normal progenitor cells, their relationship with each other, that is, synchronous expression and abnormal changes in the intensities of antigen [removed]underexpression or overexpression) compared to normal progenitors.[30] [37] [38] [57] [58] The DfN approach requires (1) an antibody panel with appropriate combinations of markers, (2) well-standardized and reproducible processing protocol and instrument setup, (3) an appropriately designed and updated MRD analysis template, and (4) adequate training and experience with detailed knowledge of immunophenotypic patterns of normal and regenerating BM progenitor cells. Thus, the approach to MFC-MRD depends on knowledge of normal patterns/sequences of various antigens and normal levels of their expression. This approach does not require knowledge of the immunophenotype of leukemic blasts at diagnosis and hence, the stability of the diagnostic LAIP during therapy may not affect the MRD results. [Fig. 2] demonstrates the normal antigen expression pattern of antigen expression of commonly used markers in the AML MRD panel. A cluster of events (cells) showing deviation or difference from the normal antigen expression patterns can be considered abnormal blasts and part of MRD.[57] [58] [59] [60] Usually, the immunophenotype of these cells coincides with the diagnostic immunophenotype. Recently, a few publications claimed the benefits of isolated evaluation of MFC-MRD based on immunophenotypic aberrancies in CD38-negative CD34-positive stem-cell-like compartment, also referred to as “leukemic stem cell–based MRD (LSC-MRD).”[45] [49] [61] [62] It is merely an extension of MFC-MRD with a specific focus on CD38-negative CD34-positive progenitors and a study of a few additional markers (e.g., CD45RA, CD366, CD371, etc.) to identify more aberrancies. The data supporting its added value in predicting survival outcomes are limited and future studies are needed to provide robust data to incorporate in clinical practice.
Gating Strategy
The initial part of the gating strategy for AML MRD follows a similar approach to that for immunophenotypic analysis for leukemia diagnosis. It starts with doublet discrimination using a scatter plot of FSC-H versus FSC-A, followed by exclusion of debris/checking for viability using FSC/SSC scatter plot, and defining the major populations based on the CD45 expression and SSC.[38] [59] [63] [64] The leukemic blasts and progenitor cells can usually be identified using the weak CD45 expression and low SSC, expression of markers of immaturity such as CD34 and CD117, and absence markers of maturation, for example, CD11b, CD14, strong CD15 expression, etc. Other lineage markers can identify different subpopulations within myeloid progenitors, such as CD13 and CD33 identify granulocytic progenitors; CD33, CD64, and CD36 identify monocytic progenitors; CD36 in the absence of myeloid and monocytic markers identifies erythroid progenitors; CD123 and HLADR identify plasmacytoid dendritic and basophil progenitors, etc. The residual disease can be detected within these populations using a combination of markers based on the LAIPs (e.g., CD7, CD11b, CD19, and CD56) and/or DfN approaches (over-/underexpression of myelomonocytic markers, e.g., CD13, CD15, CD33, CD34, CD36, CD38, CD64, CD117, CD123, and HLADR).[54] [58]
AML is an immunophenotypically heterogeneous and complex disease. Hence, to simplify the approach to MRD assessment, we have modified the gating strategy for AML MRD assessment in the Hematopathology Laboratory, Tata Memorial Centre (unpublished data). According to our strategy (shown in [Fig. 3]), mononucleated cells (MNC) are gated after the exclusion of granulocytes with high side scatter using a combination of CD38 and SSC. After the exclusion of CD45 bright lymphocytes and monocytes, this MNC progenitor region is divided into three compartments utilizing a combination of CD34 and CD117: (1) MNC with CD34-positive expression, (2) MNC with CD117 positive but CD34 negative, and (3) MNC negative for both CD34 and CD117 expressions ([Fig. 3]). This approach allows a focused assessment of progenitor cells within these three compartments. This approach emphasizes particular compartments based on the LAIP of CD34 and CD117. For example, if the leukemic blasts were CD34 negative and CD117 positive, then one can give more emphasis on that compartment. As shown in [Supp. Table 2] (available in the online version), these three compartments contain fractions of subpopulations of progenitor cells differentiating to their mature forms. These populations and their maturation patterns are highlighted in [Fig. 2]. Based on these normal maturation patterns, the progenitor cells from these three compartments are studied for DfN aberrancies and LAIPs including aberrant expression of lymphoid-associated markers such as CD7, CD19, and CD56 [removed][Fig. 4]). [Fig. 4] demonstrates examples of some AML MRD detected in CD34 + , CD117 + CD34–, and CD117-CD34–compartments from different patients. It has to be noted that a small subset of normal myeloid progenitor cells, especially in regenerating BM, usually show weak and heterogeneous expression of lymphoid markers such as CD2, CD4, CD7, and CD56.
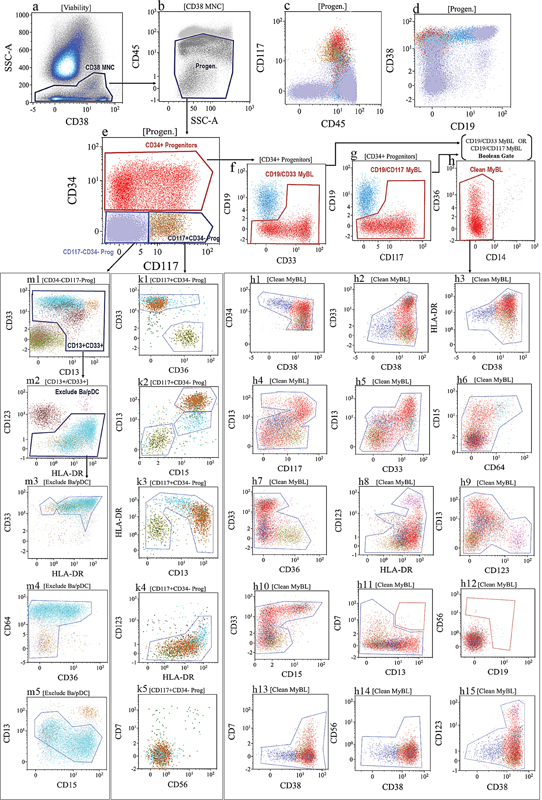
| Figure 3:The gating strategy for acute myeloid leukemia (AML) minimal residual disease (MRD) used in Tata Memorial Centre, Mumbai. The mononucleated cells (MNC) were gated after the exclusion of granulocytes with high side scatter using a combination of CD38 versus SSC (dot plot [a]). After the exclusion of CD45 bright lymphocytes and monocytes, the MNC progenitor cells were gated using a “Progen” gate (dot plot [b]). Cells from “Progen” gate were divided into three compartments utilizing a combination of CD34 and CD117: (1) CD34+ progenitors, (2) CD117+ but CD34–progenitors, and (3) CD34–and CD117–progenitors (dot plot [e]). Normal B-cell precursors are excluded from CD34+ progenitors using a Boolean gate using a combination of CD19/CD33 MyBL (dot plot [f]) “OR” CD19/CD117 MyBL (dot plot [g]). These nonlymphoid CD34+ progenitors were further cleaned (Clean MyBL gate) after removing a few CD14 + CD36+ monocytes with nonspecific binding (dot plot [h]). These progenitors gated with “Clean MyBL gate” were studied for asynchronous and aberrant maturation patterns using a combination of various markers (shown in dot plots [h1] to [h15]) based on their normal maturation patterns (shown in [Fig. 2]). Dot plots [k1] to [k5] show the distribution of CD117 +/CD34–progenitors and their patterns to study asynchronous and aberrant maturation patterns. Dot plots [m3] to [m5] showed the distribution of CD117–/CD34–progenitors after excluding CD13/CD33 negative (dot plot [m1]) and basophils (brown dots)/ plasmacytoid dendritic cells (pink dots; dot plot [m2]). Note: [Fig. 4] is a representative example of the AML MRD approach used by the author. Demonstration of a complete approach is not possible due to limited space for figures.

| Figure 4:Dot plots (A–H) show some examples of AML MRD detected in the CD34+ progenitor compartment, (I–L) in CD117+CD34- progenitor compartment and (M–Q) in CD117-CD34- compartment from different patients. AML MRD is indicated with black dots in all dot plots except in dot plots (B) and (G). Here, MRD is indicated with dark blue dots showing aberrant CD56 [removed]B) and CD33 over[removed]G) in CD38-negative stem cells.
Similarly, myeloid markers, such as CD13 and CD33, may show clustering in their expression in regenerating BM samples. Hence, it is essential to give enough consideration to this finding before labeling it an abnormal expression or LAIP. [Table 1] highlights common immunophenotypic aberrancies and LAIPs, which have been useful in MRD detection in our experience (unpublished data).
|
CD45 vs. SSC progenitor compartment |
Different from normal (DfN) |
Leukemia associated immunophenotype (LAIP) |
|
|---|---|---|---|
|
Myeloid lineage associated |
Lymphoid lineage associated |
||
|
CD34 + + |
CD203c–and HLADR– |
CD34 + ++ |
CD7 + +[a] CD13 + +CD36– |
|
HLADR + + and CD117– |
CD34 +/− |
CD7+ CD38–or HLADR– |
|
|
CD13 + +, CD117 + , and CD33– |
CD15 + , CD64–, CD13– |
CD56 + +[b] and/or CD7+ |
|
|
CD13–and CD33 + + CD15+ |
CD15 + , CD64 + , CD13– |
CD56+ CD38- |
|
|
CD13+ and CD33 + + CD117– |
CD123 + +CD117 + ++ |
CD19 + , CD13–/+ , CD56+ |
|
|
CD13 + + and CD15 |
SS HLADR– |
CD22 + CD117+ |
|
|
CD123 + +CD38– |
SS CD117– |
CD2 + +[c] |
|
|
SS CD38– |
CD117–and CD56+ or CD7+ |
CD5 + +[c] |
|
|
CD33 + + and CD38– |
CD11b+ |
||
|
HLADR + + and CD38– |
|||
|
CD117–/+ HLADR– |
|||
|
CD71-CD38– |
|||
|
CD34-CD117 + + |
CD33 + ++ |
CD123 + +CD33 + ++ |
CD56 + ++ |
|
HLADR + + +/CD33 + ++ |
CD203c-HLADR–CD13 + + |
CD7+ |
|
|
CD64 + +, CD36+ |
CD13–and CD33 + + CD15+ |
||
|
CD33 + +CD36–/+ and CD123 + + |
|||
|
CD34-CD117– |
CD33 + + + , CD36–/+ , CD123 + +, and CD14– |
CD64 + +, CD36 + , and HLADR–CD14– |
CD56 + ++ and CD33 +/CD13 +/CD64+ |
|
CD13-CD15–CD33 + + and CD14– |
HLADR + + +/CD45+ |
CD7+ and CD33 +/CD13 +/CD64+ |
|
|
CD64 + +, CD36+ and CD33–/+ and CD14– |
CD33 + +HLADR–CD64– |
||
|
CD13–, CD64 + , CD36 + +, and HLADR + ++ |
CD33 + HLADR– and CD36 + +CD42b+ |
||
References
- 1 Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015; 373 (12) 1136-1152
- 2 Pessach I, Spyropoulos T, Lamprianidou E, Kotsianidis I. MRD monitoring by multiparametric flow cytometry in AML: is it time to incorporate immune parameters?. Cancers (Basel) 2022; 14 (17) 4294
- 3 Pratz KW, Jonas BA, Pullarkat V. et al. Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol 2022; 40 (08) 855-865
- 4 Rossi G, Giambra V, Minervini MM. et al. Leukemia-associated immunophenotypes subdivided in “categories of specificity” improve the sensitivity of minimal residual disease in predicting relapse in acute myeloid leukemia. Cytometry B Clin Cytom 2020; 98 (03) 216-225
- 5 Khoury JD, Solary E, Abla O. et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 2022; 36 (07) 1703-1719
- 6 Cheson BD, Cassileth PA, Head DR. et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol 1990; 8 (05) 813-819
- 7 O'Donnell MR, Appelbaum F, Bishop M, Estey EH, Grever M, Maslak P. The National Comprehensive Cancer Network. NCCN acute leukemia practice guidelines. Oncology (Williston Park) 1996; 10 (11, Suppl): 205-221
- 8 Appelbaum FR, Baer MR, Carabasi MH. et al; National Comprehensive Cancer Network. NCCN Practice Guidelines for acute myelogenous leukemia. Oncology (Williston Park) 2000; 14 (11A): 53-61
- 9 Cheson BD, Bennett JM, Kopecky KJ. et al; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21 (24) 4642-4649
- 10 Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2004; 22 (16) 3432-3433
- 11 Inaba H, Coustan-Smith E, Cao X. et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol 2012; 30 (29) 3625-3632
- 12 Loken MR, Alonzo TA, Pardo L. et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood 2012; 120 (08) 1581-1588
- 13 Brodersen LE, Gerbing RB, Pardo ML. et al. Morphologic remission status is limited compared to ΔN flow cytometry: a Children's Oncology Group AAML0531 report. Blood Adv 2020; 4 (20) 5050-5061
- 14 Chen X, Cherian S. Role of minimal residual disease testing in acute myeloid leukemia. Clin Lab Med 2021; 41 (03) 467-483
- 15 Shook D, Coustan-Smith E, Ribeiro RC, Rubnitz JE, Campana D. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma 2009; 9 (Suppl 3): S281-S285
- 16 Vedula RS, Lindsley RC. Measurement of residual disease in acute myeloid leukemia. Curr Hematol Malig Rep 2017; 12 (06) 574-581
- 17 Freeman SD, Jovanovic JV, Grimwade D. Development of minimal residual disease-directed therapy in acute myeloid leukemia. Semin Oncol 2008; 35 (04) 388-400
- 18 Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol 2010; 22 (06) 656-663
- 19 Freeman SD, Virgo P, Couzens S. et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 2013; 31 (32) 4123-4131
- 20 Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”?. Blood 2014; 124 (23) 3345-3355
- 21 Buccisano F, Dillon R, Freeman SD, Venditti A. Role of minimal (measurable) residual disease assessment in older patients with acute myeloid leukemia. Cancers (Basel) 2018; 10 (07) 215
- 22 Freeman SD, Hills RK, Virgo P. et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 2018; 36 (15) 1486-1497
- 23 Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia 2017; 31 (07) 1482-1490
- 24 Paterno G, Del Principe MI, Venditti A. Detection and management of acute myeloid leukemia measurable residual disease: is it standard of care?. Curr Opin Hematol 2020; 27 (02) 81-87
- 25 Schuurhuis GJ, Heuser M, Freeman S. et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018; 131 (12) 1275-1291
- 26 DiNardo CD, Luger SM. Beyond morphology: minimal residual disease detection in acute myeloid leukemia. Curr Opin Hematol 2012; 19 (02) 82-88
- 27 Jacobsohn DA, Loken MR, Fei M. et al. Outcomes of measurable residual disease in pediatric acute myeloid leukemia before and after hematopoietic stem cell transplant: validation of difference from normal flow cytometry with chimerism studies and Wilms tumor 1 gene expression. Biol Blood Marrow Transplant 2018; 24 (10) 2040-2046
- 28 Freeman SD, Hills RK, Russell NH. et al; UK NCRI AML Trial Group; HOVON AML Trial Group. Induction response criteria in acute myeloid leukaemia: implications of a flow cytometric measurable residual disease negative test in refractory adults. Br J Haematol 2019; 186 (01) 130-133
- 29 Heuser M, Freeman SD, Ossenkoppele GJ. et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2021; 138 (26) 2753-2767
- 30 Chen X, Wood BL. Monitoring minimal residual disease in acute leukemia: technical challenges and interpretive complexities. Blood Rev 2017; 31 (02) 63-75
- 31 Thol F, Gabdoulline R, Liebich A. et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018; 132 (16) 1703-1713
- 32 Heuser M, Heida B, Büttner K. et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv 2021; 5 (09) 2294-2304
- 33 Simoes C, Paiva B, Martínez-Cuadrón D. et al. Measurable residual disease in elderly acute myeloid leukemia: results from the PETHEMA-FLUGAZA phase 3 clinical trial. Blood Adv 2021; 5 (03) 760-770
- 34 Caballero-Velázquez T, Pérez-López O, Yeguas Bermejo A. et al. Prognostic value of measurable residual disease in patients with AML undergoing HSCT: a multicenter study. Cancers (Basel) 2023; 15 (05) 1609
- 35 Fuda F, Chen W. Minimal/measurable residual disease detection in acute leukemias by multiparameter flow cytometry. Curr Hematol Malig Rep 2018; 13 (06) 455-466
- 36 Zhou Y, Othus M, Walter RB, Estey EH, Wu D, Wood BL. Deep NPM1 sequencing following allogeneic hematopoietic cell transplantation improves risk assessment in adults with NPM1-mutated AML. Biol Blood Marrow Transplant 2018; 24 (08) 1615-1620
- 37 Roloff GW, Lai C, Hourigan CS, Dillon LW. Technical Advances in the Measurement of Residual Disease in Acute Myeloid Leukemia. J Clin Med 2017; 6 (09) 87
- 38 Tettero JM, Freeman S, Buecklein V. et al. Technical aspects of flow cytometry-based measurable residual disease quantification in acute myeloid leukemia: experience of the European LeukemiaNet MRD working party. HemaSphere 2021; 6 (01) e676
- 39 Juul-Dam KL, Ommen HB, Nyvold CG. et al. Measurable residual disease assessment by qPCR in peripheral blood is an informative tool for disease surveillance in childhood acute myeloid leukaemia. Br J Haematol 2020; 190 (02) 198-208
- 40 Zhang YW, Su L, Tan YH. et al. Measurable residual disease detected by flow cytometry independently predicts prognoses of NPM1-mutated acute myeloid leukemia. Ann Hematol 2023; 102 (02) 337-347
- 41 McGowan F P, Hyter D S, Cui W, Plummer RM, Godwin AK, Zhang D. P FM. Comparison of flow cytometry and next-generation sequencing in minimal residual disease monitoring of acute myeloid leukemia: one institute's practical clinical experience. Int J Lab Hematol 2022; 44 (01) 118-126
- 42 Patkar N, Kakirde C, Shaikh AF. et al. Clinical impact of panel-based error-corrected next generation sequencing versus flow cytometry to detect measurable residual disease (MRD) in acute myeloid leukemia (AML). Leukemia 2021; 35 (05) 1392-1404
- 43 Fajtova M, Babusikova O. Immunophenotype characterization of hematopoietic stem cells, progenitor cells restricted to myeloid lineage and their leukemia counterparts. Neoplasma 2010; 57 (05) 392-400
- 44 Pettit K, Stock W, Walter RB. Incorporating measurable (“minimal”) residual disease-directed treatment strategies to optimize outcomes in adults with acute myeloid leukemia. Leuk Lymphoma 2016; 57 (07) 1527-1533
- 45 Wouters R, Cucchi D, Kaspers GJ, Schuurhuis GJ, Cloos J. Relevance of leukemic stem cells in acute myeloid leukemia: heterogeneity and influence on disease monitoring, prognosis and treatment design. Expert Rev Hematol 2014; 7 (06) 791-805
- 46 Farge T, Saland E, de Toni F. et al. Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discov 2017; 7 (07) 716-735
- 47 Cloos J, Harris JR, Janssen JJWM. et al. Comprehensive protocol to sample and process bone marrow for measuring measurable residual disease and leukemic stem cells in acute myeloid leukemia. J Vis Exp 2018; ;( (133) 56386
- 48 Jaddaoui S, Bencharef H, Lamchahab M, Quessar A, Oukkache B. Prognostic impact and phenotype of residual acute myeloid leukemia stem cells. Clin Lab 2022;68(06):
- 49 Khaldoyanidi SK, Hindoyan A, Stein A, Subklewe M. Leukemic stem cells as a target for eliminating acute myeloid leukemia: gaps in translational research. Crit Rev Oncol Hematol 2022; 175: 103710
- 50 Röhnert MA, Kramer M, Schadt J. et al. Reproducible measurable residual disease detection by multiparametric flow cytometry in acute myeloid leukemia. Leukemia 2022; 36 (09) 2208-2217
- 51 Kalina T, Flores-Montero J, van der Velden VH. et al; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012; 26 (09) 1986-2010
- 52 Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol 2010; 23 (03) 379-390
- 53 Baer MR, Stewart CC, Dodge RK. et al. High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood 2001; 97 (11) 3574-3580
- 54 Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T, Kern W. Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom 2004; 62 (01) 25-38
- 55 Zelezníková T, Babusíková O. The impact of cell heterogeneity and immunophenotypic changes on monitoring minimal residual disease in acute myeloid leukemia. Neoplasma 2006; 53 (06) 500-506
- 56 Langebrake C, Brinkmann I, Teigler-Schlegel A. et al. Immunophenotypic differences between diagnosis and relapse in childhood AML: implications for MRD monitoring. Cytometry B Clin Cytom 2005; 63 (01) 1-9
- 57 Xiao W, Petrova-Drus K, Roshal M. Optimal measurable residual disease testing for acute myeloid leukemia. Surg Pathol Clin 2019; 12 (03) 671-686
- 58 Wood BL. Acute myeloid leukemia minimal residual disease detection: the difference from normal approach. Curr Protoc Cytom 2020; 93 (01) e73
- 59 Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol 2013; 999: 123-136
- 60 Zhou Y, Wood BL. Methods of detection of measurable residual disease in AML. Curr Hematol Malig Rep 2017; 12 (06) 557-567
- 61 Bruserud Ø, Aasebø E, Hernandez-Valladares M, Tsykunova G, Reikvam H. Therapeutic targeting of leukemic stem cells in acute myeloid leukemia: the biological background for possible strategies. Expert Opin Drug Discov 2017; 12 (10) 1053-1065
- 62 Canali A, Vergnolle I, Bertoli S. et al. Prognostic impact of unsupervised early assessment of bulk and leukemic stem cell measurable residual disease in acute myeloid leukemia. Clin Cancer Res 2023; 29 (01) 134-142
- 63 Tembhare PR, Subramanian Pg Bertolli, Ghogale S. et al. A high-sensitivity 10-color flow cytometric minimal residual disease assay in B-lymphoblastic leukemia/lymphoma can easily achieve the sensitivity of 2-in-106 and is superior to standard minimal residual disease assay: a study of 622 patients. Cytometry B Clin Cytom 2020; 98 (01) 57-67
- 64 Tembhare PR, Chatterjee G, Khanka T. et al. Eleven-marker 10-color flow cytometric assessment of measurable residual disease for T-cell acute lymphoblastic leukemia using an approach of exclusion. Cytometry B Clin Cytom 2021; 100 (04) 421-433
- 65 Aanei CM, Veyrat-Masson R, Selicean C. et al. Database-guided analysis for immunophenotypic diagnosis and follow-up of acute myeloid leukemia with recurrent genetic abnormalities. Front Oncol 2021; 11: 746951
- 66 Piñero P, Morillas M, Gutierrez N. et al. Identification of leukemia-associated immunophenotypes by databaseguided flow cytometry provides a highly sensitive and reproducible strategy for the study of measurable residual disease in acute myeloblastic leukemia. Cancers (Basel) 2022; 14 (16) 4010
- 67 Zeijlemaker W, Kelder A, Cloos J, Schuurhuis GJ. Immunophenotypic detection of measurable residual (stem cell) disease using LAIP approach in acute myeloid leukemia. Curr Protoc Cytom 2019; 91 (01) e66
- 68 Buccisano F, Palmieri R, Piciocchi A. et al. Clinical relevance of an objective flow cytometry approach based on limit of detection and limit of quantification for measurable residual disease assessment in acute myeloid leukemia. A post-hoc analysis of the GIMEMA AML1310 trial. Haematologica 2022; 107 (12) 2823-2833
- 69 Paras G, Morsink LM, Othus M. et al. Conditioning intensity and peritransplant flow cytometric MRD dynamics in adult AML. Blood 2022; 139 (11) 1694-1706
- 70 Walter RB, Buckley SA, Pagel JM. et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122 (10) 1813-1821
- 71 Norkin M, Katragadda L, Zou F. et al. Minimal residual disease by either flow cytometry or cytogenetics prior to an allogeneic hematopoietic stem cell transplant is associated with poor outcome in acute myeloid leukemia. Blood Cancer J 2017; 7 (12) 634
- 72 Liu J, Ma R, Liu YR. et al. The significance of peri-transplantation minimal residual disease assessed by multiparameter flow cytometry on outcomes for adult AML patients receiving haploidentical allografts. Bone Marrow Transplant 2019; 54 (04) 567-577
- 73 Nagler A, Baron F, Labopin M. et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant 2021; 56 (01) 218-224
- 74 Fasan O. Using minimal (measurable) residual disease assessments to guide decision-making for timing of allogeneic transplantation in acute myeloid leukemia. Curr Opin Hematol 2019; 26 (06) 413-420
- 75 Guolo F, Di Grazia C, Minetto P. et al. Pre-transplant minimal residual disease assessment and transplant-related factors predict the outcome of acute myeloid leukemia patients undergoing allogeneic stem cell transplantation. Eur J Haematol 2021; 107 (05) 573-582
- 76 Klyuchnikov E, Christopeit M, Badbaran A. et al. Role of pre-transplant MRD level detected by flow cytometry in recipients of allogeneic stem cell transplantation with AML. Eur J Haematol 2021; 106 (05) 606-615
- 77 Oran B, Jorgensen JL, Marin D. et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica 2017; 102 (01) 110-117
- 78 Shen X, Pan J, Qi C. et al. Impact of pre-transplantation minimal residual disease (MRD) on the outcome of Allogeneic hematopoietic stem cell transplantation for acute leukemia. Hematology 2021; 26 (01) 295-300
- 79 Rossi G, Carella AM, Minervini MM. et al. Optimal time-points for minimal residual disease monitoring change on the basis of the method used in patients with acute myeloid leukemia who underwent allogeneic stem cell transplantation: a comparison between multiparameter flow cytometry and Wilms' tumor 1 expression. Leuk Res 2015; 39 (02) 138-143
- 80 Zhou Y, Othus M, Araki D. et al. Pre- and post-transplant quantification of measurable (“minimal”) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016; 30 (07) 1456-1464
- 81 Klyuchnikov E, Badbaran A, Massoud R. et al. Post-transplantation day +100 minimal residual disease detection rather than mixed chimerism predicts relapses after allogeneic stem cell transplantation for intermediate-risk acute myelogenous leukemia patients undergoing transplantation in complete remission. Transplant Cell Ther 2022; 28 (07) 374.e1-374.e9
- 82 Meur GL, Plesa A, Larcher MV. et al. Impact on outcome of minimal residual disease after hematopoietic stem cell transplantation with fludarabine, amsacrine, and cytosine arabinoside-busulfan conditioning: a retrospective monocentric study. Transplant Cell Ther 2023; 29 (01) 38.e1-38.e9
- 83 Winters AC, Bosma G, Abbott D. et al. Outcomes are similar after allogeneic hematopoietic stem cell transplant for newly diagnosed acute myeloid leukemia patients who received venetoclax + azacitidine versus intensive chemotherapy. Transplant Cell Ther 2022; 28 (10) 694.e1-694.e9
- 84 Soh KT, Conway A, Liu X, Wallace PK. Development of a 27-color panel for the detection of measurable residual disease in patients diagnosed with acute myeloid leukemia. Cytometry A 2022; 101 (11) 970-983
- 85 Maag AH, Swanton H, Kull M, Vegi NM, Feuring M. Immunophenotypical profiling of myeloid neoplasms with erythroid predominance using mass cytometry (CyTOF). Cytometry A 2023; 1003 (07) 551-562
Address for correspondence
Prashant R. Tembhare, MDClinician Scientist and ProfessorRoom 18, Hematopathology Laboratory, CCE Building, ACTREC, Sector 22, Navi Mumbai 410210, MaharashtraIndiaEmail: docprt@gmail.comPublication History
Article published online:
27 November 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 : (A–I) Normal maturation stages of CD34+ hematopoietic progenitors and their differentiation toward various sublineages with antigen expression patterns. CLP, common lymphoid progenitors; CM/LP, common myeloid and lymphoid progenitors; CMP, common myeloid progenitors; GMP, granulocyte–macrophage (monocyte) progenitors; HSC, hematopoietic stem cells; MCP, Mast cell precursors; MPP, multipotent progenitors; MEP, megakaryocyte erythrocyte progenitors; pDC-P, plasmacytoid dendritic cell precursors.

| Figure 2:Normal maturation patterns of CD34+ hematopoietic progenitors with their differentiation toward various sublineages using commonly used antigen expression. Each dot plot shows the differentiation of hematopoietic stem cells (dark blue dots) toward the respective committed precursors using a combination of different markers. The maturation sequences are shown with black arrows. Red dots indicate granulocytic differentiation, cyan blue dots indicate monocytic differentiation, olive green dots indicate erythroid differentiation, light blue dots indicate B-cell precursors, pink dots indicate plasmacytoid dendritic cell precursors, and brown dots indicate basophil precursors. The gray color dots indicate the background mature mononuclear cells from a commonly used CD45 versus SSC progenitor gate (shown in [Fig. 1]). Abbreviations: Ba, basophils; BCP, B-cell precursors; CMP, common myeloid progenitors; HSC, hematopoietic stem cells; MPP, multipotent progenitors; PMy, precursor myeloid cells; PMo, precursor monocytes; PNB; precursor normoblasts; pDC, plasmacytoid dendritic cell.

| Figure 3:The gating strategy for acute myeloid leukemia (AML) minimal residual disease (MRD) used in Tata Memorial Centre, Mumbai. The mononucleated cells (MNC) were gated after the exclusion of granulocytes with high side scatter using a combination of CD38 versus SSC (dot plot [a]). After the exclusion of CD45 bright lymphocytes and monocytes, the MNC progenitor cells were gated using a “Progen” gate (dot plot [b]). Cells from “Progen” gate were divided into three compartments utilizing a combination of CD34 and CD117: (1) CD34+ progenitors, (2) CD117+ but CD34–progenitors, and (3) CD34–and CD117–progenitors (dot plot [e]). Normal B-cell precursors are excluded from CD34+ progenitors using a Boolean gate using a combination of CD19/CD33 MyBL (dot plot [f]) “OR” CD19/CD117 MyBL (dot plot [g]). These nonlymphoid CD34+ progenitors were further cleaned (Clean MyBL gate) after removing a few CD14 + CD36+ monocytes with nonspecific binding (dot plot [h]). These progenitors gated with “Clean MyBL gate” were studied for asynchronous and aberrant maturation patterns using a combination of various markers (shown in dot plots [h1] to [h15]) based on their normal maturation patterns (shown in [Fig. 2]). Dot plots [k1] to [k5] show the distribution of CD117 +/CD34–progenitors and their patterns to study asynchronous and aberrant maturation patterns. Dot plots [m3] to [m5] showed the distribution of CD117–/CD34–progenitors after excluding CD13/CD33 negative (dot plot [m1]) and basophils (brown dots)/ plasmacytoid dendritic cells (pink dots; dot plot [m2]). Note: [Fig. 4] is a representative example of the AML MRD approach used by the author. Demonstration of a complete approach is not possible due to limited space for figures.

| Figure 4:Dot plots (A–H) show some examples of AML MRD detected in the CD34+ progenitor compartment, (I–L) in CD117+CD34- progenitor compartment and (M–Q) in CD117-CD34- compartment from different patients. AML MRD is indicated with black dots in all dot plots except in dot plots (B) and (G). Here, MRD is indicated with dark blue dots showing aberrant CD56 [removed]B) and CD33 over[removed]G) in CD38-negative stem cells.
References
- 1 Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015; 373 (12) 1136-1152
- 2 Pessach I, Spyropoulos T, Lamprianidou E, Kotsianidis I. MRD monitoring by multiparametric flow cytometry in AML: is it time to incorporate immune parameters?. Cancers (Basel) 2022; 14 (17) 4294
- 3 Pratz KW, Jonas BA, Pullarkat V. et al. Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol 2022; 40 (08) 855-865
- 4 Rossi G, Giambra V, Minervini MM. et al. Leukemia-associated immunophenotypes subdivided in “categories of specificity” improve the sensitivity of minimal residual disease in predicting relapse in acute myeloid leukemia. Cytometry B Clin Cytom 2020; 98 (03) 216-225
- 5 Khoury JD, Solary E, Abla O. et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 2022; 36 (07) 1703-1719
- 6 Cheson BD, Cassileth PA, Head DR. et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol 1990; 8 (05) 813-819
- 7 O'Donnell MR, Appelbaum F, Bishop M, Estey EH, Grever M, Maslak P. The National Comprehensive Cancer Network. NCCN acute leukemia practice guidelines. Oncology (Williston Park) 1996; 10 (11, Suppl): 205-221
- 8 Appelbaum FR, Baer MR, Carabasi MH. et al; National Comprehensive Cancer Network. NCCN Practice Guidelines for acute myelogenous leukemia. Oncology (Williston Park) 2000; 14 (11A): 53-61
- 9 Cheson BD, Bennett JM, Kopecky KJ. et al; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21 (24) 4642-4649
- 10 Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2004; 22 (16) 3432-3433
- 11 Inaba H, Coustan-Smith E, Cao X. et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol 2012; 30 (29) 3625-3632
- 12 Loken MR, Alonzo TA, Pardo L. et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood 2012; 120 (08) 1581-1588
- 13 Brodersen LE, Gerbing RB, Pardo ML. et al. Morphologic remission status is limited compared to ΔN flow cytometry: a Children's Oncology Group AAML0531 report. Blood Adv 2020; 4 (20) 5050-5061
- 14 Chen X, Cherian S. Role of minimal residual disease testing in acute myeloid leukemia. Clin Lab Med 2021; 41 (03) 467-483
- 15 Shook D, Coustan-Smith E, Ribeiro RC, Rubnitz JE, Campana D. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma 2009; 9 (Suppl 3): S281-S285
- 16 Vedula RS, Lindsley RC. Measurement of residual disease in acute myeloid leukemia. Curr Hematol Malig Rep 2017; 12 (06) 574-581
- 17 Freeman SD, Jovanovic JV, Grimwade D. Development of minimal residual disease-directed therapy in acute myeloid leukemia. Semin Oncol 2008; 35 (04) 388-400
- 18 Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol 2010; 22 (06) 656-663
- 19 Freeman SD, Virgo P, Couzens S. et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 2013; 31 (32) 4123-4131
- 20 Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”?. Blood 2014; 124 (23) 3345-3355
- 21 Buccisano F, Dillon R, Freeman SD, Venditti A. Role of minimal (measurable) residual disease assessment in older patients with acute myeloid leukemia. Cancers (Basel) 2018; 10 (07) 215
- 22 Freeman SD, Hills RK, Virgo P. et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 2018; 36 (15) 1486-1497
- 23 Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia 2017; 31 (07) 1482-1490
- 24 Paterno G, Del Principe MI, Venditti A. Detection and management of acute myeloid leukemia measurable residual disease: is it standard of care?. Curr Opin Hematol 2020; 27 (02) 81-87
- 25 Schuurhuis GJ, Heuser M, Freeman S. et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018; 131 (12) 1275-1291
- 26 DiNardo CD, Luger SM. Beyond morphology: minimal residual disease detection in acute myeloid leukemia. Curr Opin Hematol 2012; 19 (02) 82-88
- 27 Jacobsohn DA, Loken MR, Fei M. et al. Outcomes of measurable residual disease in pediatric acute myeloid leukemia before and after hematopoietic stem cell transplant: validation of difference from normal flow cytometry with chimerism studies and Wilms tumor 1 gene expression. Biol Blood Marrow Transplant 2018; 24 (10) 2040-2046
- 28 Freeman SD, Hills RK, Russell NH. et al; UK NCRI AML Trial Group; HOVON AML Trial Group. Induction response criteria in acute myeloid leukaemia: implications of a flow cytometric measurable residual disease negative test in refractory adults. Br J Haematol 2019; 186 (01) 130-133
- 29 Heuser M, Freeman SD, Ossenkoppele GJ. et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2021; 138 (26) 2753-2767
- 30 Chen X, Wood BL. Monitoring minimal residual disease in acute leukemia: technical challenges and interpretive complexities. Blood Rev 2017; 31 (02) 63-75
- 31 Thol F, Gabdoulline R, Liebich A. et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018; 132 (16) 1703-1713
- 32 Heuser M, Heida B, Büttner K. et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv 2021; 5 (09) 2294-2304
- 33 Simoes C, Paiva B, Martínez-Cuadrón D. et al. Measurable residual disease in elderly acute myeloid leukemia: results from the PETHEMA-FLUGAZA phase 3 clinical trial. Blood Adv 2021; 5 (03) 760-770
- 34 Caballero-Velázquez T, Pérez-López O, Yeguas Bermejo A. et al. Prognostic value of measurable residual disease in patients with AML undergoing HSCT: a multicenter study. Cancers (Basel) 2023; 15 (05) 1609
- 35 Fuda F, Chen W. Minimal/measurable residual disease detection in acute leukemias by multiparameter flow cytometry. Curr Hematol Malig Rep 2018; 13 (06) 455-466
- 36 Zhou Y, Othus M, Walter RB, Estey EH, Wu D, Wood BL. Deep NPM1 sequencing following allogeneic hematopoietic cell transplantation improves risk assessment in adults with NPM1-mutated AML. Biol Blood Marrow Transplant 2018; 24 (08) 1615-1620
- 37 Roloff GW, Lai C, Hourigan CS, Dillon LW. Technical Advances in the Measurement of Residual Disease in Acute Myeloid Leukemia. J Clin Med 2017; 6 (09) 87
- 38 Tettero JM, Freeman S, Buecklein V. et al. Technical aspects of flow cytometry-based measurable residual disease quantification in acute myeloid leukemia: experience of the European LeukemiaNet MRD working party. HemaSphere 2021; 6 (01) e676
- 39 Juul-Dam KL, Ommen HB, Nyvold CG. et al. Measurable residual disease assessment by qPCR in peripheral blood is an informative tool for disease surveillance in childhood acute myeloid leukaemia. Br J Haematol 2020; 190 (02) 198-208
- 40 Zhang YW, Su L, Tan YH. et al. Measurable residual disease detected by flow cytometry independently predicts prognoses of NPM1-mutated acute myeloid leukemia. Ann Hematol 2023; 102 (02) 337-347
- 41 McGowan F P, Hyter D S, Cui W, Plummer RM, Godwin AK, Zhang D. P FM. Comparison of flow cytometry and next-generation sequencing in minimal residual disease monitoring of acute myeloid leukemia: one institute's practical clinical experience. Int J Lab Hematol 2022; 44 (01) 118-126
- 42 Patkar N, Kakirde C, Shaikh AF. et al. Clinical impact of panel-based error-corrected next generation sequencing versus flow cytometry to detect measurable residual disease (MRD) in acute myeloid leukemia (AML). Leukemia 2021; 35 (05) 1392-1404
- 43 Fajtova M, Babusikova O. Immunophenotype characterization of hematopoietic stem cells, progenitor cells restricted to myeloid lineage and their leukemia counterparts. Neoplasma 2010; 57 (05) 392-400
- 44 Pettit K, Stock W, Walter RB. Incorporating measurable (“minimal”) residual disease-directed treatment strategies to optimize outcomes in adults with acute myeloid leukemia. Leuk Lymphoma 2016; 57 (07) 1527-1533
- 45 Wouters R, Cucchi D, Kaspers GJ, Schuurhuis GJ, Cloos J. Relevance of leukemic stem cells in acute myeloid leukemia: heterogeneity and influence on disease monitoring, prognosis and treatment design. Expert Rev Hematol 2014; 7 (06) 791-805
- 46 Farge T, Saland E, de Toni F. et al. Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discov 2017; 7 (07) 716-735
- 47 Cloos J, Harris JR, Janssen JJWM. et al. Comprehensive protocol to sample and process bone marrow for measuring measurable residual disease and leukemic stem cells in acute myeloid leukemia. J Vis Exp 2018; ;( (133) 56386
- 48 Jaddaoui S, Bencharef H, Lamchahab M, Quessar A, Oukkache B. Prognostic impact and phenotype of residual acute myeloid leukemia stem cells. Clin Lab 2022;68(06):
- 49 Khaldoyanidi SK, Hindoyan A, Stein A, Subklewe M. Leukemic stem cells as a target for eliminating acute myeloid leukemia: gaps in translational research. Crit Rev Oncol Hematol 2022; 175: 103710
- 50 Röhnert MA, Kramer M, Schadt J. et al. Reproducible measurable residual disease detection by multiparametric flow cytometry in acute myeloid leukemia. Leukemia 2022; 36 (09) 2208-2217
- 51 Kalina T, Flores-Montero J, van der Velden VH. et al; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012; 26 (09) 1986-2010
- 52 Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol 2010; 23 (03) 379-390
- 53 Baer MR, Stewart CC, Dodge RK. et al. High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood 2001; 97 (11) 3574-3580
- 54 Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T, Kern W. Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom 2004; 62 (01) 25-38
- 55 Zelezníková T, Babusíková O. The impact of cell heterogeneity and immunophenotypic changes on monitoring minimal residual disease in acute myeloid leukemia. Neoplasma 2006; 53 (06) 500-506
- 56 Langebrake C, Brinkmann I, Teigler-Schlegel A. et al. Immunophenotypic differences between diagnosis and relapse in childhood AML: implications for MRD monitoring. Cytometry B Clin Cytom 2005; 63 (01) 1-9
- 57 Xiao W, Petrova-Drus K, Roshal M. Optimal measurable residual disease testing for acute myeloid leukemia. Surg Pathol Clin 2019; 12 (03) 671-686
- 58 Wood BL. Acute myeloid leukemia minimal residual disease detection: the difference from normal approach. Curr Protoc Cytom 2020; 93 (01) e73
- 59 Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol 2013; 999: 123-136
- 60 Zhou Y, Wood BL. Methods of detection of measurable residual disease in AML. Curr Hematol Malig Rep 2017; 12 (06) 557-567
- 61 Bruserud Ø, Aasebø E, Hernandez-Valladares M, Tsykunova G, Reikvam H. Therapeutic targeting of leukemic stem cells in acute myeloid leukemia: the biological background for possible strategies. Expert Opin Drug Discov 2017; 12 (10) 1053-1065
- 62 Canali A, Vergnolle I, Bertoli S. et al. Prognostic impact of unsupervised early assessment of bulk and leukemic stem cell measurable residual disease in acute myeloid leukemia. Clin Cancer Res 2023; 29 (01) 134-142
- 63 Tembhare PR, Subramanian Pg Bertolli, Ghogale S. et al. A high-sensitivity 10-color flow cytometric minimal residual disease assay in B-lymphoblastic leukemia/lymphoma can easily achieve the sensitivity of 2-in-106 and is superior to standard minimal residual disease assay: a study of 622 patients. Cytometry B Clin Cytom 2020; 98 (01) 57-67
- 64 Tembhare PR, Chatterjee G, Khanka T. et al. Eleven-marker 10-color flow cytometric assessment of measurable residual disease for T-cell acute lymphoblastic leukemia using an approach of exclusion. Cytometry B Clin Cytom 2021; 100 (04) 421-433
- 65 Aanei CM, Veyrat-Masson R, Selicean C. et al. Database-guided analysis for immunophenotypic diagnosis and follow-up of acute myeloid leukemia with recurrent genetic abnormalities. Front Oncol 2021; 11: 746951
- 66 Piñero P, Morillas M, Gutierrez N. et al. Identification of leukemia-associated immunophenotypes by databaseguided flow cytometry provides a highly sensitive and reproducible strategy for the study of measurable residual disease in acute myeloblastic leukemia. Cancers (Basel) 2022; 14 (16) 4010
- 67 Zeijlemaker W, Kelder A, Cloos J, Schuurhuis GJ. Immunophenotypic detection of measurable residual (stem cell) disease using LAIP approach in acute myeloid leukemia. Curr Protoc Cytom 2019; 91 (01) e66
- 68 Buccisano F, Palmieri R, Piciocchi A. et al. Clinical relevance of an objective flow cytometry approach based on limit of detection and limit of quantification for measurable residual disease assessment in acute myeloid leukemia. A post-hoc analysis of the GIMEMA AML1310 trial. Haematologica 2022; 107 (12) 2823-2833
- 69 Paras G, Morsink LM, Othus M. et al. Conditioning intensity and peritransplant flow cytometric MRD dynamics in adult AML. Blood 2022; 139 (11) 1694-1706
- 70 Walter RB, Buckley SA, Pagel JM. et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122 (10) 1813-1821
- 71 Norkin M, Katragadda L, Zou F. et al. Minimal residual disease by either flow cytometry or cytogenetics prior to an allogeneic hematopoietic stem cell transplant is associated with poor outcome in acute myeloid leukemia. Blood Cancer J 2017; 7 (12) 634
- 72 Liu J, Ma R, Liu YR. et al. The significance of peri-transplantation minimal residual disease assessed by multiparameter flow cytometry on outcomes for adult AML patients receiving haploidentical allografts. Bone Marrow Transplant 2019; 54 (04) 567-577
- 73 Nagler A, Baron F, Labopin M. et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant 2021; 56 (01) 218-224
- 74 Fasan O. Using minimal (measurable) residual disease assessments to guide decision-making for timing of allogeneic transplantation in acute myeloid leukemia. Curr Opin Hematol 2019; 26 (06) 413-420
- 75 Guolo F, Di Grazia C, Minetto P. et al. Pre-transplant minimal residual disease assessment and transplant-related factors predict the outcome of acute myeloid leukemia patients undergoing allogeneic stem cell transplantation. Eur J Haematol 2021; 107 (05) 573-582
- 76 Klyuchnikov E, Christopeit M, Badbaran A. et al. Role of pre-transplant MRD level detected by flow cytometry in recipients of allogeneic stem cell transplantation with AML. Eur J Haematol 2021; 106 (05) 606-615
- 77 Oran B, Jorgensen JL, Marin D. et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica 2017; 102 (01) 110-117
- 78 Shen X, Pan J, Qi C. et al. Impact of pre-transplantation minimal residual disease (MRD) on the outcome of Allogeneic hematopoietic stem cell transplantation for acute leukemia. Hematology 2021; 26 (01) 295-300
- 79 Rossi G, Carella AM, Minervini MM. et al. Optimal time-points for minimal residual disease monitoring change on the basis of the method used in patients with acute myeloid leukemia who underwent allogeneic stem cell transplantation: a comparison between multiparameter flow cytometry and Wilms' tumor 1 expression. Leuk Res 2015; 39 (02) 138-143
- 80 Zhou Y, Othus M, Araki D. et al. Pre- and post-transplant quantification of measurable (“minimal”) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016; 30 (07) 1456-1464
- 81 Klyuchnikov E, Badbaran A, Massoud R. et al. Post-transplantation day +100 minimal residual disease detection rather than mixed chimerism predicts relapses after allogeneic stem cell transplantation for intermediate-risk acute myelogenous leukemia patients undergoing transplantation in complete remission. Transplant Cell Ther 2022; 28 (07) 374.e1-374.e9
- 82 Meur GL, Plesa A, Larcher MV. et al. Impact on outcome of minimal residual disease after hematopoietic stem cell transplantation with fludarabine, amsacrine, and cytosine arabinoside-busulfan conditioning: a retrospective monocentric study. Transplant Cell Ther 2023; 29 (01) 38.e1-38.e9
- 83 Winters AC, Bosma G, Abbott D. et al. Outcomes are similar after allogeneic hematopoietic stem cell transplant for newly diagnosed acute myeloid leukemia patients who received venetoclax + azacitidine versus intensive chemotherapy. Transplant Cell Ther 2022; 28 (10) 694.e1-694.e9
- 84 Soh KT, Conway A, Liu X, Wallace PK. Development of a 27-color panel for the detection of measurable residual disease in patients diagnosed with acute myeloid leukemia. Cytometry A 2022; 101 (11) 970-983
- 85 Maag AH, Swanton H, Kull M, Vegi NM, Feuring M. Immunophenotypical profiling of myeloid neoplasms with erythroid predominance using mass cytometry (CyTOF). Cytometry A 2023; 1003 (07) 551-562


 PDF
PDF  Views
Views  Share
Share

