Primary mediastinal seminoma; resistance and relapse: An aggressive entity
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 309-312
DOI: DOI: 10.4103/0971-5851.125252
Abstract
Seminoma is one of the most radio and chemo sensitive tumors. Aggressive variant of primary mediastinal seminoma (PMS) are rarely described in the literature. We here discuss an interesting case of young male (22 years) presenting with superior vena cava syndrome due to PMS that showed poor response to conventional platinum based chemotherapy and radiotherapy. Furthermore, with a short disease free interval (one year) relapsed at unusual sites of bone and soft-tissue deposits in check. This emphasizes the importance of identifying methods to pre-screen such aggressive variants for more effective treatment options.
Keywords
Bone metastasis - chemoresistance - extra-gonadal germ cell tumor - mediastinal seminoma - radio-resistance - soft-tissue metastasisPublication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Seminoma is one of the most radio and chemo sensitive tumors. Aggressive variant of primary mediastinal seminoma (PMS) are rarely described in the literature. We here discuss an interesting case of young male (22 years) presenting with superior vena cava syndrome due to PMS that showed poor response to conventional platinum based chemotherapy and radiotherapy. Furthermore, with a short disease free interval (one year) relapsed at unusual sites of bone and soft-tissue deposits in check. This emphasizes the importance of identifying methods to pre-screen such aggressive variants for more effective treatment options.
INTRODUCTION
The primary mediastinal seminoma (PMS) is a rare tumor. Usually, these tumors are slow growing and have limited potential to metastasis.[1] This report presents a rare case of PMS with several rare and interesting features. This includes younger age of presentation, placental alkaline phosphatase (PLAP) negative seminoma, chemo (cisplatin based) and radio-resistance, and unusual sites of metastasis at recurrence. Bone metastasis is a rare occurrence with seminoma while the presence of soft- tissue metastasis is yet to be described in association with PMS.
CASE REPORT
A 22-year-old male presented with a history of progressive dyspnea, cough, hoarseness of voice, and unquantified weight loss for the last 6 months in Feb 2010. On clinical examination, there were signs of superior vena cava syndrome, with 5 cm × 6 cm left supraclavicular mass. The whole body computed tomography (CT) scan showed heterogeneously enhancing 12 cm × 8.2 cm × 4.2 cm firms soft-tissue mass in the mediastinum encasing ascending aorta, arch of aorta, right main pulmonary artery, and proximal bronchi, infiltrating superior vena cava [Figure 1]. The ultrasound of scrotum was unremarkable. The biopsy of left supraclavicular mass was consistent for extragonadal seminoma with imunohistochemistry positive for C-kit and negative for leucocyte common antigen, epithelial membrane antigen and PLAP. The serum lactate dehydrogenase (LDH) was raised to 1963 U/L (normal range 100-190 U/L) while β- human chorionic gonadotropin (β- HCG <1.20 mlU/ml) and alpha fetoprotein AFP (2.73 ng/ml) were normal. The baseline complete blood counts, renal and liver function tests were found to be normal.
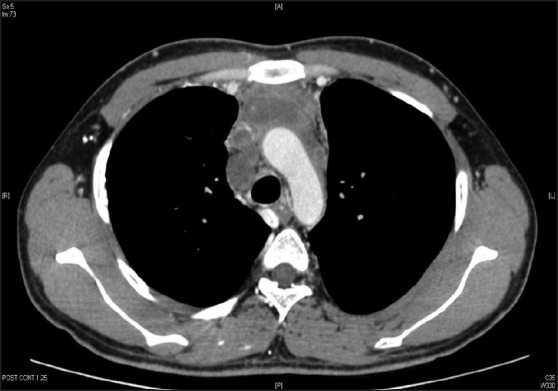
| Fig. 1 Computed tomography chest showing primary mediastinal seminoma with involvement of major vessels; especially invasion of superior vena cava
The patient received chemotherapy comprising of bleomycin, etoposide, and cisplatin (BEP; Bleomycin 18 U/m2 IV, Etoposide 100 mg/m2, Cisplatin 20 mg/m2) 3 weekly for four cycles until July 2010. The patient tolerated chemotherapy well with no dose reductions. There was a serial reduction in serum LDH values. The response assessment CT showed residual disease of 5.8 cm × 4 × 4 cm and elevated serum LDH of 221 U/L. To consolidate the partial response and vascular involvement precluding surgery; external beam radiation to a dose of 30 Gy in 17 fractions over 3 weeks was given to the mediastinum and supraclavicular region. The 6 MV photons with the tomotherapy (high precision arc intensity modulated radiation with image guidance) were used to irradiate the planning target volume, which included clinical target volume (CTV) with a margin of 5 mm for set-up error. The CTV included gross residual tumor as seen on planning CT with 5 mm margin. The post-radiation positron emission tomography-CT (PET-CT) showed complete metabolic regression of the disease and the normal serum LDH. Patient was on regular follow-up with tumor markers and CT thorax abdomen pelvis every 3 monthly.
At 1 year patient presented with rapidly progressing right side cheek swelling (5 cm × 6 cm) and progressive lower backache. On examination, there was local tenderness in cheek swelling and in the region of 5th lumbar vertebrae. The CT showed a soft-tissue mass involving subcutaneous plane of right cheek of 6 cm × 6 cm × 4 cm [Figure 2]; increased size of mediastinal mass (10 cm × 8 cm × 6 cm) and multiple lytic lesions in ribs. The fine needle aspiration cytology from check swelling showed cells consistent with the metastatic seminoma collaborated with raised serum LDH values (353U/L). The serum β-HCG and AFP were normal. The technetium-99 m- methylene diphosphonate MDP bone scintigraphy revealed multiple metastases in ribs and 5th lumbar vertebra.
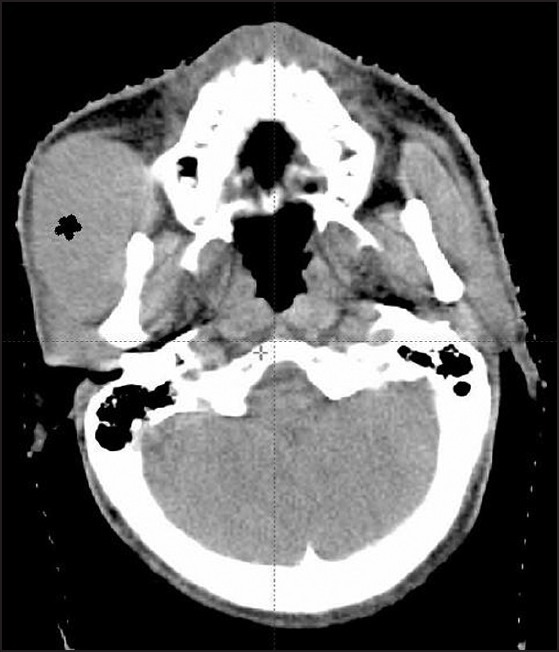
| Fig. 2 Computed tomography head neck showing soft-tissue deposit in check (*)
After discussion in institutional joint tumor board meeting keeping the view of disease status, poor performance status and patient wishes; he was offered short course palliative radiotherapy to right face and lumbar spine with the supportive care. Review after 4 weeks showed poor pain relief and progressive disease. Taking an overall view of patient performance status advanced disease, distant geographical location, challenged socio-economic status and discussions with the patient and family members; further anti-neoplastic therapy was precluded and best supportive care was offered. Patient succumbed to progression of the disease shortly.
DISCUSSION
Primary extragonadal germ cell tumors (EGGCT) constitute a small subset of 2-5% of all germ cell tumors;[1] and PMS include only 10% of EGGCT.[1] In reported literature, PMS presents at a young age (median 33 years) with dyspnea, cough, fever, weight loss, and Superior vena cava obstruction SVCO symptoms in decreasing order.[1] The current case is one of the rare PMS presenting at much younger age of 22 years with SVCO, which would equally contend for a diagnosis of lymphoma.
In one large series of EGGCT with over 600 patients, post-cisplatin based chemotherapy overall response rate was 92%, and refractoriness to cisplatin based chemotherapy was the most important factor associated with the poor outcomes. In PMS only two out of 25 patients showed viable tumor in residual mass post-chemotherapy. In this patient due to mediastinal great vessels invasion surgery was precluded; though radiation achieved a complete metabolic response of a very short duration. Another notable unusual feature was, that PET-CT after completion of treatment showed complete metabolic remission suggesting a possibility longer disease free survival contrary to that patient developed local relapse and distant failure in a very short period of time.[2] Although, negative predictive value of PET-CT is reported to be 90% in literature the probability of missing residual disease, which recurred after a short interval of 1 year cannot be ignored.[3] Hence, development of better follows-up strategies including introspection of (Fludeoxyglucose) FDG-PET to identify the relapse are required.[4]
The recurrence after chemotherapy and radiotherapy in PMS has been rarely associated with malignant transformations to sarcomas. The exclusion of such an event would have been desirous with biopsy and imunohistochemistry marker but was excluded as per patient wishes and socio-economic issues.[5] Although, the results of the second line chemotherapy especially with high-dose regimens with stem cell rescue have been encouraging in the literature recently; but were again excluded after considering patient and disease factors.[6]
The most important aspect from clinical profile of this patient includes unusual sites of metastasis at the time of recurrence. Despite the finding that soft-tissue comprises approximately 55% of our body mass, hematogenous metastases to these areas are rare. Direct extension of a primary tumor to soft- tissue is a much more common event than distant soft-tissue metastases.[7] Autopsy studies have also addressed the incidence of secondary malignancies involving soft-tissue sites. One of the large autyopsy series concluded that secondary tumors were actually much more common than primary malignant tumors of soft-tissue.[8] Plaza et al. analyzed total 118 cases of soft-tissue metastasis, in 27.11% of cases the metastasis to soft-tissue was the initial manifestation of the disease leading to the subsequent identification of the primary tumor elsewhere. Most commonly reported malignancies resulting in distant soft-tissue metastases are lung, kidney, and colon carcinoma. Whereas, only one of 118 cases had testicular cancer as primary.[7] Six patients with primary testicular malignancy have been described in literature with soft-tissue metastasis.[7,8,9,10] Four had seminoma histology with most common sites of deposits in the abdominal wall, back, thigh, chest, and shoulder. In all the four patients, these metastases were present at the time of recurrence. This is the first report where PMS had metastasis at soft-tissue, also cheek as the site of metastasis has never been described before in association with PMS.[11]
The other unusual site of metastasis in this patient was bone. Until now, only three patients of PMS with bone metastasis at presentation have been described in the literature.[1,6] None of the cases developed bone metastasis at recurrence. Hence soft-tissue and bone metastasis together at relapse in PMS with a short disease free interval is a rare previously unreported clinical entity. The early relapse and unusual site of metastasis suggests aggressive histology. The metastasis at both sites were unresponsive to radiotherapy is also quite unusual for seminomas.
The 5-10% of all seminomas is anaplastic histology associated with increased frequency of metastasis and locally advanced stage. Although 30% of patients who die from seminoma have anaplastic morphology but stage-to-stage, treatment outcomes for patients with classic versus anaplastic seminoma have been similar.[12] The expression of genes (dopamine receptor D1 and family with sequence similarity 71, member F2 (FAM71F2) and microsatellite instability have been associated with chemoresistance and increased metastatic seminoma.[13,14] However, whether the PMS have the similar genes pre-disposing to chemoresistance and aggressive spread to unusual sites is unknown. The present case indicates that such patients are prone for early relapse and routine follow-up strategies like serum markers and conventional CT scans at a regular interval may be inadequate. Treatment strategies like Imatinib in metastatic seminoma and other targeted agents have recently shown promise.[15]
To summarize this is a rare case of PMS with earlier age of presentation with features of SVCO. The poor response to platinum based chemotherapy is uncommon and a poor prognostic marker although there was a complete “metabolic and biochemical response” post-chemo-radiotherapy. However, this was followed by an early relapse with unusual sites of metastasis. PMS is a rare tumor and aggressive variants are even rarer. Genetic markers predisposing to aggressive behavior, chemo/radio resistance, early relapse, and unusual sites of metastasis should be identified. Such patients although rare must be identified for improved treatment modalities and follow-up strategies to detect recurrences and metastasis early.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Computed tomography chest showing primary mediastinal seminoma with involvement of major vessels; especially invasion of superior vena cava

| Fig. 2 Computed tomography head neck showing soft-tissue deposit in check (*)


 PDF
PDF  Views
Views  Share
Share

